ЬтФПФкШн
ЁОЬтФПЁППЦбЇМвЛ§МЋЬНЫїаТММЪѕЖдCO2НјаазлКЯРћгУ, CO2ПЩгУРДКЯГЩЕЭЬМЬўЁЃ
CO2(g) +4H2(g) ![]() CH4(g) + 2H2O(g) ІЄH= a kJ/mol
CH4(g) + 2H2O(g) ІЄH= a kJ/mol
(1)вбжЊЃКЂй4H2ЃЈgЃЉ+ 2O2ЃЈgЃЉ=4H2OЃЈgЃЉ ЁїH=-967.2kJ/molЃЎ
ЂкCH4(g) + 2O2(g) =CO2(g) + 2H2O(g) ІЄH=-802.0 kJ/molЃЎ
ЧыЛиД№ЃКЂйЂкетСНИіЗДгІдкШШСІбЇЩЯЧїЪЦОљКмДѓЃЌЦфдвђЪЧ__________________ЃЛ a=____________kJ/molЁЃ
(2)дкЬхЛ§ЮЊ1LЕФУмБеИеадШнЦїжаЃЌГфШы4mol H2КЭ1mol CO2ЃЌВтЕУЮТЖШЖдCO2ЕФЦНКтзЊЛЏТЪКЭДпЛЏМСДпЛЏаЇТЪЕФгАЯьШчЭМ1ЫљЪОЁЃ
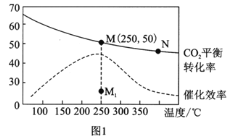
ЂйвбжЊMЕузмбЙЮЊ1MPaЃЌИУЗДгІдкДЫЮТЖШЯТЕФЦНКтГЃЪ§Kp=______MPa-2ЁЃ(KpЪЧгУЦНКтЗжбЙДњЬцЦНКтХЈЖШБэЪОЕФЛЏбЇЦНКтГЃЪ§ЃЌЦјЬхЗжбЙ=ЦјЬхзмбЙЁСЬхЛ§ЗжЪ§ЁЃЃЉ
ЂкгћдіМгЖўбѕЛЏЬМЕФЦНКтзЊЛЏТЪЃЌПЩВЩШЁЕФДыЪЉга__________ЁЃ
AЃЎЭЈШыЖшадЦјЬх BЃЎЬсИпЮТЖШ
CЃЎдіМгЖўбѕЛЏЬМХЈЖШ DЃЎдіМгЧтЦјХЈЖШ
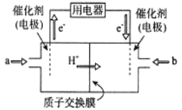
ЂлЯТСаЫЕЗЈе§ШЗЕФЪЧ_________ЁЃ
AЃЎЦНКтГЃЪ§ДѓаЁЃКKN>KM
BЃЎЦфЫћЬѕМўВЛБфЃЌШєВЛЪЙгУДпЛЏМСЃЌдђ250Ёц ЪБCO2ЕФЦНКтзЊЛЏТЪПЩФмЮЛгкЕуM1
CЃЎЭМ1жаMЕуЪБЃЌМзЭщЕФЬхЛ§ЗжЪ§ЮЊ12.5%
DЃЎЕБбЙЧПЛђn( H2)/n(CO2)ВЛБфЪБОљПЩжЄУїЛЏбЇЗДгІвбДяЕНЦНКтзДЬЌ
(3)аТаЭИпаЇЕФМзЭщШМСЯЕчГиЙЄзїЪБзмЗДгІЪНЃКCH4+2O2=CO2+2H2OЁЃ
ЂйИУЕчГиЕФИКМЋЪЧ___________(ЬюaЛђb)ЃЌЂкИКМЋЕчМЋЗДгІЪНЮЊ___________ЃЛ
ЁОД№АИЁПСНИіЗДгІЖМЗХГіДѓСПЕФШШ -165.2 1 D C a CH4+2H2O-8e-=CO2+8H+
ЁОНтЮіЁП
(1)вбжЊЃКЂй4H2(g)+ 2O2(g)=4H2O(g) ЁїH=-967.2kJ/molЃЌЂкCH4(g) + 2O2(g) =CO2(g) + 2H2O(g) ІЄH=-802.0 kJ/molЃЌгЩИЧЫЙЖЈТЩПЩжЊЂй-ЂкЕУCO2(g) +4H2(g) ![]() CH4(g) + 2H2O(g)ЃЌгЩДЫМЦЫу ІЄHЃЛ
CH4(g) + 2H2O(g)ЃЌгЩДЫМЦЫу ІЄHЃЛ
(2)ЂйвбжЊMЕузмбЙЮЊ1MPaЃЌCO2ЕФзЊЛЏТЪЮЊ50%ЃЌдђЃК

![]()
ЦНКтЬхЯЕжаCO2ЕФЬхЛ§ЗжЪ§ЮЊ![]() =
=![]() ЃЌH2ЕФЬхЛ§ЗжЪ§ЮЊ
ЃЌH2ЕФЬхЛ§ЗжЪ§ЮЊ![]() =
=![]() ЃЌCH4ЕФЬхЛ§ЗжЪ§ЮЊ
ЃЌCH4ЕФЬхЛ§ЗжЪ§ЮЊ![]() =
=![]() ЃЌH2OЕФЬхЛ§ЗжЪ§ЮЊ
ЃЌH2OЕФЬхЛ§ЗжЪ§ЮЊ![]() ЃЌгЩДЫМЦЫуИУЗДгІдкДЫЮТЖШЯТЕФЦНКтГЃЪ§KpЃЛ
ЃЌгЩДЫМЦЫуИУЗДгІдкДЫЮТЖШЯТЕФЦНКтГЃЪ§KpЃЛ
ЂкгћдіМгЖўбѕЛЏЬМЕФЦНКтзЊЛЏТЪЃЌГЃЭЈЙ§ПижЦЗДгІЬѕМўДйНјЦНКте§ЯђвЦЖЏМДПЩЃЛ
ЂлAЃЎЗДгІЪЧЗХШШЗДгІЃЌЮТЖШЩ§ИпЦНКтФцЯђНјааЃЛ
BЃЎИљОнЭМЯѓЗжЮіЃЛ
CЃЎЭМжаMЕуЪБЖўбѕЛЏЬМЕФзЊЛЏТЪ50%ЃЌНсКЯЛЏбЇЦНКтШ§ааМЦЫуСаЪНЕУЕНЃЛ
DЃЎЛьКЯЦјЬхЕФжЪСПВЛБфЃЌЬхЛ§ВЛБфЃЌЫљвдЛьКЯЦјЬхЕФУмЖШЪМжеВЛБфЃЛ
(3)ШМСЯЕчГиЭЈO2ЕФМЋЮЊе§МЋЃЌИКМЋЩЯЗЂЩњбѕЛЏЗДгІЃЌЧвЭтЕчТЗЕчзггЩИКМЋСїЯђе§МЋЁЃ
(1)вђЂйЂкетСНИіЗДгІСНИіЗДгІЖМЗХГіДѓСПЕФШШЃЌдђДгШШСІбЇНЧЖШЗжЮіЃЌетСНИіЗДгІЧїЪЦОљКмДѓЃЛвбжЊЃКЂй4H2(g)+ 2O2(g)=4H2O(g) ЁїH=-967.2kJ/molЃЌЂкCH4(g) + 2O2(g) =CO2(g) + 2H2O(g) ІЄH=-802.0 kJ/molЃЌгЩИЧЫЙЖЈТЩПЩжЊЂй-ЂкЕУCO2(g) +4H2(g) ![]() CH4(g) + 2H2O(g)ЃЌдђІЄH=(-967.2kJ/mol)-(-802.0 kJ/mol)=-165.2 kJ/mol= a kJ/molЃЌМДa=-165.2ЃЛ
CH4(g) + 2H2O(g)ЃЌдђІЄH=(-967.2kJ/mol)-(-802.0 kJ/mol)=-165.2 kJ/mol= a kJ/molЃЌМДa=-165.2ЃЛ
(2)ЂйвбжЊMЕузмбЙЮЊ1MPaЃЌCO2ЕФзЊЛЏТЪЮЊ50%ЃЌдђЃК

![]()
ЦНКтЬхЯЕжаCO2ЕФЬхЛ§ЗжЪ§ЮЊ![]() =
=![]() ЃЌH2ЕФЬхЛ§ЗжЪ§ЮЊ
ЃЌH2ЕФЬхЛ§ЗжЪ§ЮЊ![]() =
=![]() ЃЌCH4ЕФЬхЛ§ЗжЪ§ЮЊ
ЃЌCH4ЕФЬхЛ§ЗжЪ§ЮЊ![]() =
=![]() ЃЌH2OЕФЬхЛ§ЗжЪ§ЮЊ
ЃЌH2OЕФЬхЛ§ЗжЪ§ЮЊ![]() ЃЌгЩДЫМЦЫуИУЗДгІдкДЫЮТЖШЯТЕФЦНКтГЃЪ§Kp=
ЃЌгЩДЫМЦЫуИУЗДгІдкДЫЮТЖШЯТЕФЦНКтГЃЪ§Kp=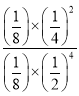 MPa-2=1MPa-2ЃЛ
MPa-2=1MPa-2ЃЛ
ЂкAЃЎдкКуЮТКуШнЬѕМўЯТЃЌЭЈШыЖшадЦјЬхЃЌбЙЧПдіДѓЃЌЕЋВЛИФБфЗДгІЬхЯЕИїЮяжЪЕФХЈЖШЃЌВЛИФБфЗДгІЫйТЪЃЌЦНКтВЛвЦЖЏЃЌЖўбѕЛЏЬМЕФЦНКтзЊЛЏТЪВЛБфЃЌЙЪAДэЮѓЃЛ
BЃЎе§ЗДгІЗХШШЃЌЬсИпЮТЖШЃЌЦНКтФцЯђвЦЖЏЃЌЖўбѕЛЏЬМЕФЦНКтзЊЛЏТЪНЕЕЭЃЌЙЪBДэЮѓЃЛ
CЃЎдіМгЖўбѕЛЏЬМХЈЖШЃЌЦНКте§ЯђвЦЖЏЃЌH2ЕФзЊЛЏТЪдіМгЃЌЖјЖўбѕЛЏЬМЕФЦНКтзЊЛЏТЪНЕЕЭЃЌЙЪCДэЮѓЃЛ
DЃЎдіМгЧтЦјХЈЖШЃЌЦНКте§ЯђвЦЖЏЃЌH2ЕФзЊЛЏТЪНЕЕЭЃЌЖјЖўбѕЛЏЬМЕФЦНКтзЊЛЏТЪдіМгЃЌЙЪDе§ШЗЃЛ
ЙЪД№АИЮЊDЃЛ
ЂлAЃЎЩ§ИпЮТЖШЖўбѕЛЏЬМЕФЦНКтзЊЛЏТЪМѕЕЭЃЌдђЩ§ЮТЦНКтФцЯђвЦЖЏЃЌЫљвдMЛЏбЇЦНКтГЃЪ§ДѓгкNЃЌЙЪAДэЮѓЃЛ
BЃЎгЩЭМЯѓПЩжЊЃЌЦфЫћЬѕМўВЛБфЃЌШєВЛЪЙгУДпЛЏМСЃЌдђ250ЁцЪБCO2ЕФЦНКтзЊЛЏТЪПЩФмЮЛгкЕуMЃЌЙЪBДэЮѓЃЛ
CЃЎЭМ1жаMЕуЪБЃЌгЩЂйЕФНтЮіжЊМзЭщЕФЬхЛ§ЗжЪ§ЮЊ![]() =12.5%ЃЌЙЪCе§ШЗЃЛ
=12.5%ЃЌЙЪCе§ШЗЃЛ
DЃЎЛьКЯЦјЬхЕФжЪСПВЛБфЃЌЬхЛ§ВЛБфЃЌЫљвдЛьКЯЦјЬхЕФУмЖШЪМжеВЛБфЃЌЫљвдВЛФмИљОнЛьКЯЦјЬхЕФУмЖШРДХаЖЯЛЏбЇЗДгІЪЧЗёДяЕНЦНКтзДЬЌЃЌЙЪDДэЮѓЃЛ
ЙЪД№АИЮЊCЃЛ
(3)ЂйгаМзЭщШМСЯЕчГизмЗДгІЪНЃКCH4+2O2=CO2+2H2OПЩжЊЃЌЭЈO2ЕФМЋЮЊе§МЋЃЌЧвЭтЕчТЗЕчзггЩИКМЋСїЯђе§МЋЃЌдђaМЋЮЊИКМЋЃЛ
ЂкИКМЋЩЯCH4ЗЂЩњбѕЛЏЗДгІЃЌЦфЕчМЋЗДгІЪНЮЊCH4+2H2O-8e-=CO2+8H+ЁЃ

 ПЮПЮСЗНЫеЯЕСаД№АИ
ПЮПЮСЗНЫеЯЕСаД№АИ УћХЦжабЇПЮЪБзївЕЯЕСаД№АИ
УћХЦжабЇПЮЪБзївЕЯЕСаД№АИ УїЬьНЬг§ПЮЪБЬибЕЯЕСаД№АИ
УїЬьНЬг§ПЮЪБЬибЕЯЕСаД№АИ еуНаТПЮГЬШ§ЮЌФПБъВтЦРПЮЪБЬибЕЯЕСаД№АИ
еуНаТПЮГЬШ§ЮЌФПБъВтЦРПЮЪБЬибЕЯЕСаД№АИ