��Ŀ����
2��Co��Cu��Zn���ǹ���Ԫ�أ�����Ϊ����ԭ���γɶ����������1�����в�����Ϊ�������λ�����C
A��H2O B��NH3 C��CH4 D��Cl-
��2���������ʾʽд����ˮ��NH3������ˮ���Ӽ��γɵĿ��ܴ��ڵ����N-H��O��O-H��N��
��3��CuԪ�ؿ��γ�[Cu��NH3��4]SO4�����д��ڵĻ�ѧ�������Т٢ۢݣ�����ţ���
����λ�����ڽ��������ۼ��Թ��ۼ����ܷǼ��Թ��ۼ� �����Ӽ��������
��4����[Cu��NH3��4]2+���жԳƵĿռ乹�ͣ��ҵ����е�����NH3������Cl-ȡ��ʱ���ܵõ����ֲ�ͬ�ṹ�IJ����[Cu��NH3��4]2+�Ŀռ乹��Ϊa������ĸ����
a��ƽ�������� b���������� c�������� d��V��
������ͭ��Һ����εμӰ�ˮ���������ȳ�����ɫ�������������ܽ��γ�����ɫ����Һ��д������ɫ�����ܽ�����ӷ���ʽ��Cu��OH��2+4NH3•H2O=[Cu��NH3��4]2++2OH-+4H2O��
��5����CoCl2�ܽ���ˮ��Ӱ�ˮֱ�������ɵ�Co��OH��2�������ܽ���ټ����Ӱ�ˮ��ʹ֮����[Co��NH3��6]2+����ʱ����Һ��ͨ��������õ��IJ�������һ�ֵ���ɿ���CoCl3•5NH3��ʾ���ѷ������CoCl3•5NH3����ˮ����������������Һ��������AgCl���������ⶨÿ1mol CoCl3•5NH3ֻ����2mol AgCl����д����ʾ�������ṹ�Ľṹ��ʽ��[Co��NH3��5Cl]Cl2��
���� ��1������Ϊ�������λ������躬�й¶Ե��ӣ�CH4�¶Ե��ӣ�
��2��NԪ����OԪ�صĵ縺�Զ���ǿ����ͬ�����е�Nԭ�ӡ�Oԭ����Hԭ��֮�䶼�����γ������
��3��[Cu��NH3��4]SO4���������Ӽ��γ����Ӽ���������[Cu��NH3��4]2+�к�����λ������ͬ�ǽ���Ԫ��֮���γɼ��Թ��ۼ�����
��4���γ�4����λ�������жԳƵĿռ乹�ͣ�����Ϊƽ�������λ��������壬���Ϊ�������壬[Cu��NH3��4]2+�е�����NH3������Cl-ȡ����ֻ��һ�ֽṹ��ͭ���ӿ��Ժ�һˮ�ϰ�����������ͭ�������������������ڹ����İ�ˮ�У�
��5��1mol CoCl3•5NH3ֻ����2molAgCl��˵��һ��CoCl3•5NH3�к�������Cl-��һ��ClΪ��ԭ�ӣ��ܵ���λ��Ϊ6������һ��Clԭ��Ϊ��ԭ�ӣ����Ի�����5��NH3Ϊ���壬�ݴ�ȷ���仯ѧʽ��
��� �⣺��1��A��H2O�ǹ��ۻ��������ԭ�Ӻ���ԭ���γɹ��ۼ�������ʽΪ ���й¶Ե��ӣ�������Ϊ���壬��A��ѡ��
���й¶Ե��ӣ�������Ϊ���壬��A��ѡ��
B��NH3�ǹ��ۻ������ԭ�ӷֱ���3����ԭ��ͨ��һ�Թ��õ��ӶԽ�ϣ�NH3����ʽΪ ���й¶Ե��ӣ�������Ϊ���壬��B��ѡ��
���й¶Ե��ӣ�������Ϊ���壬��B��ѡ��
C��̼ԭ��������4�����ӷֱ�����ԭ���γɹ��õ��Ӷԣ�����ʽΪ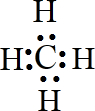 ���¶Ե��ӣ���������Ϊ���壬��Cѡ��
���¶Ե��ӣ���������Ϊ���壬��Cѡ��
D��Cl-Ϊ��ԭ�ӵõ�1�������γɵ������ӣ�����ʽΪ ���й¶Ե��ӣ�������Ϊ���壬��D��ѡ��
���й¶Ե��ӣ�������Ϊ���壬��D��ѡ��
�ʴ�Ϊ��C��
��2��NԪ����OԪ�صĵ縺�Զ���ǿ����ͬ�����е�Nԭ�ӡ�Oԭ����Hԭ��֮�䶼�����γ��������O-H��N��N-H��O��O-H��O��N-H��N����ˮ��NH3������ˮ���Ӽ��γɵĿ��ܴ��ڵ����N-H��O��O-H��N��
�ʴ�Ϊ��N-H��O��O-H��N��
��3��[Cu��NH3��4]SO4�У�[Cu��NH3��4]2+��SO42-֮��Ļ�ѧ��Ϊ���Ӽ���[Cu��NH3��4]2+��Cu2+��NH3֮��Ļ�ѧ��Ϊ��λ����N-HΪ���Թ��ۼ���[Cu��NH3��4]SO4��
�ʴ�Ϊ���٢ۢݣ�
��4���γ�4����λ�������жԳƵĿռ乹�ͣ�����Ϊƽ�������λ��������壬���Ϊ�������壬[Cu��NH3��4]2+�е�����NH3������Cl-ȡ����ֻ��һ�ֽṹ������ӦΪƽ�������Σ�
������ͭ�����������ڹ����İ�ˮ�У�������Ӧ��Cu��OH��2+4NH3•H2O=[Cu��NH3��4]2++2OH-+4H2O��
�ʴ�Ϊ��a��Cu��OH��2+4NH3•H2O=[Cu��NH3��4]2++2OH-+4H2O��
��5��1mol CoCl3•5NH3ֻ����2molAgCl��˵��һ��CoCl3•5NH3�к�������Cl-��һ��ClΪ��ԭ�ӣ��ܵ���λ��Ϊ6������һ��Clԭ��Ϊ��ԭ���⣬������5��NH3Ϊ���壬�����仯ѧʽΪ[Co��NH3��5Cl]Cl2��
�ʴ�Ϊ��[[Co��NH3��5Cl]Cl2��
���� ���⿼������ݽ�Ϊ�ۺϣ��漰����λ������������Ŀռ�Ľṹ������ﻯѧʽ�������й�֪ʶ��ע�����֪ʶ��ȫ�����գ�ע������������Ĺ��ɼ����ʣ���Ŀ�Ѷ��еȣ�

| A�� | Cl2 | B�� | SO2 | C�� | N2 | D�� | NH3 |
| A�� | 9.6g | B�� | 6.4g | C�� | 3.2g | D�� | 1.6g |
| A�� | -770 kJ/mol | B�� | -l220 kJ/mol | C�� | -1500kJ/mol | D�� | -2740kJ/mol |
| A�� | Fe2+��Mg2+��Al3+ | B�� | Mg2+��Al3+��Cu2+ | C�� | Fe3+��Cu2+��Zn2+ | D�� | Ag+��Cu2+��Zn2+ |
| A�� | ������1L0.1mol/LNH4Cl��Һ��2L0.05mol/LNH4Cl��Һ��c��NH4+����� | |
| B�� | pH=5��CH3COOH��Һ��pH=5��NH4NO3��Һ�У�c��H+����� | |
| C�� | pH=6��CH3COOH��CH3COONa���Һ�У�c��Na+��+c��OH-��-c��CH3COO-��=10-6mol/L | |
| D�� | pH=9��NaHA��Һ�У�c��Na+����c��HA-����c��A2-����c��H2A����c��OH-����c��H+�� |

| A�� | Ԫ�صķǽ����ԣ�Y��W | |
| B�� | �����ӵĻ�ԭ�ԣ�W2-��Q- | |
| C�� | ���Ӱ뾶��Y2-��Z3+ | |
| D�� | Q��W��Y��Ӧ���⻯��ķе����� |
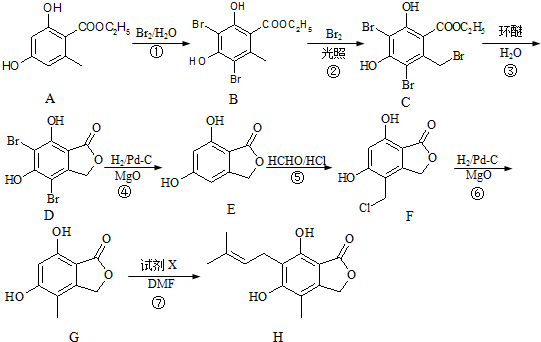
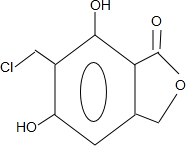 ����дһ�֣���
����дһ�֣���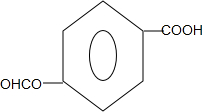 ��
��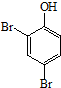 ��HCHOΪԭ���Ʊ�
��HCHOΪԭ���Ʊ� 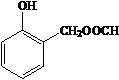 �ϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�
�ϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�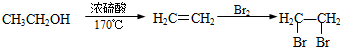
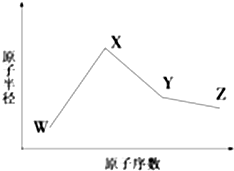 W��X��Y��Z��M��N�����ֳ����Ķ�����Ԫ�أ�����W��X��Y��Zԭ�Ӱ뾶��ԭ�������仯��ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Neԭ�ӵĺ�����������1��Y�ĵ�����һ�ֳ����İ뵼����ϣ�Z�ķǽ�������ͬ��������Ԫ������ǿ��M��ԭ��������YС1��NԪ������ϼ�����ͻ��ϼ۾���ֵ��3����
W��X��Y��Z��M��N�����ֳ����Ķ�����Ԫ�أ�����W��X��Y��Zԭ�Ӱ뾶��ԭ�������仯��ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Neԭ�ӵĺ�����������1��Y�ĵ�����һ�ֳ����İ뵼����ϣ�Z�ķǽ�������ͬ��������Ԫ������ǿ��M��ԭ��������YС1��NԪ������ϼ�����ͻ��ϼ۾���ֵ��3����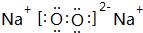 ��
��