��Ŀ����
9��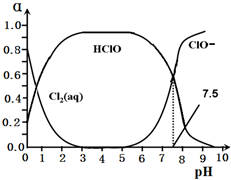 Ԫ�����ڱ��Т�A��Ԫ�صĵ��ʼ��仯�������;�㷺��
Ԫ�����ڱ��Т�A��Ԫ�صĵ��ʼ��仯�������;�㷺����1���������壨BrF3�������ں�ȼ�������ͺ�������ˮ�����������·�Ӧ��
3BrF3+5H2O��HBrO3+Br2+9HF+O2���÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ2��3��ÿ����2.24LO2����״����ת�Ƶ�����Ϊ0.6NA��
��2����ʳ����������������ؿ�Ԥ��ȱ�⣮Ϊ�˼���ʳ���еĵ���أ��ɼ������͵���-�⻯����Һ����������������Һ��������Ӧ�����ӷ���ʽ��5I-+IO3-+6CH3COOH=3I2+3H2O+6CH3COO-��
�ȳ���������ˮ��ɱ��������HClO��ɱ��������ClO-ǿ��25��ʱ����-��ˮ��ϵ�д�������ƽ���ϵ��
Cl2��g��?Cl2��aq�� ��
Cl2��aq��+H2O?HClO+H++Cl- ��
HClO?H++ClO- ��
����Cl2��aq����HClO��ClO-�ֱ�����������ռ������������pH�仯�Ĺ�ϵ��ͼ��ʾ��
��3��д��������ϵ�����ڵ���ƽ���ƽ�ⳣ������ʽ��Ki=$\frac{[{H}^{+}]•[Cl{O}^{-}]}{[HClO]}$����ͼ��֪�ó���ֵΪ10-7.5��
��4���ڸ���ϵ��c��HClO��+c��ClO-��С�� c��H+��-c��OH-�� ������ڡ���С�ڡ����ڡ�����
��5�����ȴ�������ˮʱ���ļ���ɱ��Ч���ȶ������á������������������ԭ�������¶����ߣ����ܽ⣩ƽ�� �������ƶ���Cl2��aq��Ũ�ȼ��٣�ʹ�ã���ѧƽ�⣩�������ƶ���c��HClO�����٣�ɱ��Ч����
���� ��1���ڷ�Ӧ3BrF3+5H2O=HBrO3+Br2+O2+9HF�У�����ת��������£� ���ݴ˷�����
���ݴ˷�����
��2���⻯�غ͵�����������������ܷ���������ԭ��Ӧ���ɵ⣬�������۱���ɫ��
��3��������ϵ�����ڵ���ƽ�����HClO?H++ClO-����ͼ�����ݼ��㣻
��4���κε������Һ�ж����ڵ���غ㣬�ݵ���غ������
��5��������ܽ�����¶ȵ����߶����ͣ��¶����ߣ����ܽ⣩ƽ�� �������ƶ����ݴ˷�����
��� �⣺��1���������ת�������������ԭ��ΪH2O��BrF3��������ΪBrF3��ÿ����1molO2����2molH2O��1molBrF3����ԭ����2molBrF3����������ת�Ƶ���6mol�������������ͻ�ԭ�����ʵ���֮��Ϊ2��3������2.24L����ת�Ƶ�����Ϊ0.6NA��
�ʴ�Ϊ��2��3��0.6NA��
��2������������������¾��������ԣ�����KI����������ԭ��Ӧ���ɵ��ʵ⣬��Ӧ�����ӷ���ʽΪ5I-+IO3-+6CH3COOH=3I2+3H2O+6CH3COO-�������������ɫ��
�ʴ�Ϊ����Һ������5I-+IO3-+6CH3COOH=3I2+3H2O+6CH3COO-��
��3��������ϵ�����ڵ���ƽ�����HClO?H++ClO-����ƽ�ⳣ������ʽΪ��K=$\frac{[{H}^{+}]•[Cl{O}^{-}]}{[HClO]}$����pH=7.5ʱ��c��ClO-����c��HClO����ȣ�K=c��H+��=10-7.5��
�ʴ�Ϊ��$\frac{[{H}^{+}]•[Cl{O}^{-}]}{[HClO]}$��10-7.5��
��4����ϵ�д��ڵ���غ�c��H+��=c��Cl-��+c��ClO-��+c��OH-������c��Cl-��+c��ClO-��=c��H+��-c��OH-��������ˮ��HCl��ȫ���롢HClO���ֵ��룬����c��HClO����c��Cl-��������c��HClO��+c��ClO-����c��H+��-c��OH-�����ʴ�Ϊ��С�ڣ�
��5��������ܽ�����¶ȵ����߶����ͣ��¶����ߣ����ܽ⣩ƽ�� �������ƶ���Cl2��aq��Ũ�ȼ��٣�ʹ�ã���ѧƽ�⣩�������ƶ���c��HClO�� ���٣�ɱ��Ч�����ʴ�Ϊ����¶����ߣ����ܽ⣩ƽ�� �������ƶ���Cl2��aq��Ũ�ȼ��٣�ʹ�ã���ѧƽ�⣩�������ƶ���c��HClO�� ���٣�ɱ��Ч����
���� ���⿼����������ԭ��Ӧ����������ԭ���жϼ����㡢���ӷ���ʽ��д����ѧƽ�ⳣ�������㡢ƽ���ƶ�����Ŀ�ѶȽϴ�

| A�� | pH��Ϊ4��H2SO4��NH4Cl��Һ�У�ˮ�ĵ���̶���ͬ | |
| B�� | ��pH��NaOH��Һ��NH3•H2O ϡ�ͺ�pH�ı仯����ͼ��ʾ��������I��ʾ����NaOH��Һ��ϡ�� | |
| C�� | 1mol/LNa2CO3��Һ�д��ڣ�c��Na+��=2c��CO32- ��+2c��HCO3-�� | |
| D�� | ��ij�¶ȵİ�ˮ��ͨ�����ᣬ��ˮ�ĵ��볣������ |
| A�� | 23g��NO2��N2O4��ɵĻ�������к��еĵ�ԭ����Ϊ0.5NA | |
| B�� | 1.12L��ϩ���뺬0.05NA������ӵ�CCl4��Һǡ����ȫ��Ӧ | |
| C�� | 1L0.1mol•L-1Fe2��SO4��3��Һ�У�Fe3+����ĿΪ0.2NA | |
| D�� | 7.8gNa2O2��ˮ��ȫ��Ӧʱת�Ƶĵ�����Ϊ0.2NA |
| A�� | ��п��Ƥ��ʳ��ˮ�з������ⸯʴ | |
| B�� | ���ص���������һ�����������ϻ��� | |
| C�� | ���������Դ��������������������п | |
| D�� | ԭ��صĸ����͵��ص�����������������Ӧ |
| A�� | ת�Ƶĵ�������0.1NA | B�� | ��Ӧ��Һ����������1.6g | ||
| C�� | ���ɵ������к���0.8mol���� | D�� | ��������������1.12L |
| A�� | NO3-��K+��Cl-��I- | B�� | NH4+��Na+��SO42-��Ba2+ | ||
| C�� | Na+��SO42-��HCO3-��K+ | D�� | Mg2+��Cl-��SO42-��Na+ |
 ��
��