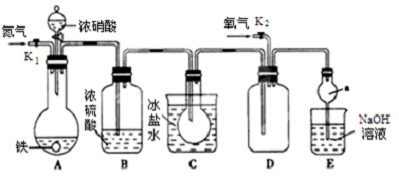
��Ŀ����
9����ˮ���ۺ����ã���������ͼ���£�
��ҵ���Դ�ʳ�Σ���������Ca2+��Mg2+���ʣ�������ʯ��ʯ��Ϊԭ�ϣ������Ʊ�Na2CO3����ش�
��1���ڴ�������ˮ�Ĺ����У��ɼ���ʯ����ʹ�����Ϊ�������������������ijɷֳ������ij��������CaCO3��Mg��OH��2��
��2����CaOͶ�뺬�д�����NH4Cl��ĸҺ�У������ɿ�ѭ��ʹ�õ�NH3���÷�Ӧ�Ļ�ѧ����ʽ��2NH4Cl+CaO=CaCl2+2NH3��+H2O��
��3����ʳ��ˮ������ͨ���������NH3�����̢�������NaHCO3����ķ�Ӧ�Ļ�ѧ����ʽ��NH3+H2O+CO2+NaCl=NaHCO3��+NH4Cl
��4��̼���ƾ���ʧˮ�������仯ʾ��ͼ���£�
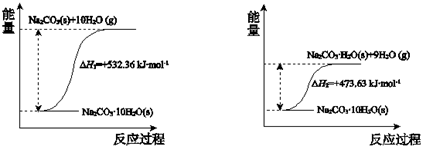
Na2CO3•H2O ��s�� ��ˮ��Ӧ���Ȼ�ѧ����ʽ��Na2CO3•H2O��s��=Na2CO3��s��+H2O��g����H=+58.73kJ•mol-1��
��5����Ʒ�����г�����NaCl��ȡa g�����������ϡ�����ַ�Ӧ�����ȡ����ɡ����գ���b g���壮��ò�Ʒ��Na2CO3������������$\frac{16��b-a��}{11a}$��100%��
��ⱥ��ʳ��ˮ��������X����Ӧ������ȡ�嵥�ʣ���ش�
��1������X�Ļ�ѧʽΪCl2����Ӧ��Ļ�ѧ����ʽΪSO2+Br2+2H2O=2HBr+H2SO4��
��2����ĸҺ��±�к��н϶��NaCl��KCl��MgCl2��MgSO4�����ʣ��ó������ⶨ��±��þԪ�صĺ�����g/L����ʵ�������Ӧ�ⶨ�������п�±��Ʒ�������Mg��OH��2������������
��3�����200kg ��������Ϊ25%�ı���ʳ��ˮ����Ũ���½���20%ʱ���ռ������������ʵ���Ϊ97.7mol��������Һ�е��������Բ��ƣ�����������һλС������
���� �����̿�֪����ˮɹ�η�������κ�ĸҺ��±������ˮ����ʯ����ʹ�����Ϊ������������̼��ơ�������þ���������˵õ�����ˮ������Ũ���õ�����ʳ��ˮ����ʳ��ˮ����ͨ��������ͨ������̼����Ӧ����̼�����ƺ��Ȼ�泥�̼�����Ʒֽ�õ�Na2CO3��ĸҺ��±Ũ����ͨ���������������ӣ��嵥�������������������ԭ��Ӧ���������HBr�����������������HBr��Ӧ�õ��壮
��1�������ӻ���̼����������ɳ�����þ���ӻ����������������ɳ�����
��2����ʯ�Һ�ˮ��Ӧ�����������ƣ��������ƺ��Ȼ�立�Ӧ���ɰ�����
��3��ʳ�Ρ�������ˮ�Ͷ�����̼������ѧ��Ӧ������̼����狀��Ȼ�泥��ݴ���д��ѧ����ʽ��
��4������̼���ƾ���ʧˮ�������仯ʾ��ͼ��֪��Na2CO3•10H2O��s��=Na2CO3��s��+10H2O��g����H=+532.36 kJ•mol-1�٣�Na2CO3•10H2O��s��=Na2CO3•H2O��s��+9H2O��g����H=+473.63 kJ•mol-1�ڣ���ϸ�˹���ɿ�֪�ɢ�-�ڵ�Na2CO3•H2O��s��=Na2CO3��s��+H2O��g����
��5������Na2CO3+2HCl=2NaCl+H2O+CO2�����м��㣻
��1�������̿�֪��XΪCl2�����������ԣ��ܽ�����������Ϊ�嵥�ʣ���ӦII�з���������������������ԭ��Ӧ��
��2���ó������ⶨ��±��þԪ�صĺ�����g/L����Ӧ֪����Һ�������MgԪ�ص�������
��3�����200kg ��������Ϊ25%�ı���ʳ��ˮ����Ũ���½���20%ʱ�����2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$Cl2��+H2��+2NaOH����μӷ�Ӧ��NaCl��Ȼ������ռ���������
��� �⣺�����̿�֪����ˮɹ�η�������κ�ĸҺ��±������ˮ����ʯ����ʹ�����Ϊ������������̼��ơ�������þ���������˵õ�����ˮ������Ũ���õ�����ʳ��ˮ����ʳ��ˮ����ͨ��������ͨ������̼����Ӧ����̼�����ƺ��Ȼ�泥�̼�����Ʒֽ�õ�Na2CO3��ĸҺ��±Ũ����ͨ���������������ӣ��嵥�������������������ԭ��Ӧ���������HBr�����������������HBr��Ӧ�õ��壮
��1������ʯ����ʹ�����Ϊ������������Ca2++CO32-=CaCO3����Mg2++2OH-=Mg��OH��2���������������ijɷֳ������ij��������CaCO3��Mg��OH��2��
�ʴ�Ϊ��CaCO3��Mg��OH��2��
��2��������NaHCO3������ĸҺ�м��������ʯ�ң������ķ�Ӧ��H2O+CaO=Ca��OH��2��Ca��OH��2+2NH4Cl=2NH3��+2H2O+CaCl2�����ղ���Ϊ�Ȼ��ơ����������а����������ã�
�ʴ�Ϊ��2NH4Cl+CaO=CaCl2+2NH3��+H2O��
��3���������̼��ˮ�е��ܽ��С��������������ˮ��������ʳ��ˮ������ͨ��NH3���ҹ��̢�������NaHCO3����ķ�Ӧ�Ļ�ѧ����ʽ��NH3+H2O+CO2+NaCl=NaHCO3��+NH4Cl��̼�����Ƶ��ܽ��С����Ӧ���Ծ�����ʽ������
�ʴ�Ϊ��NH3��NH3+H2O+CO2+NaCl=NaHCO3��+NH4Cl��
��4������̼���ƾ���ʧˮ�������仯ʾ��ͼ��֪��Na2CO3•10H2O��s��=Na2CO3��s��+10H2O��g����H=+532.36 kJ•mol-1�٣�Na2CO3•10H2O��s��=Na2CO3•H2O��s��+9H2O��g����H=+473.63 kJ•mol-1�ڣ��ɢ�-�ڵ�Na2CO3•H2O��s��=Na2CO3��s��+H2O��g����H=+58.73 kJ•mol-1��
�ʴ�Ϊ��Na2CO3•H2O��s��=Na2CO3��s��+H2O��g����H=+58.73 kJ•mol-1��
��5����Ʒ�����г�����NaCl��ȡa g�����������ϡ�����ַ�Ӧ������Na2CO3+2HCl=2NaCl+H2O+CO2������b g����ΪNaCl����̼���Ƶ����ʵ���Ϊx��
Na2CO3+2HCl=2NaCl+H2O+CO2��
1 2
x 2x
�����������117x+a-106x=b�����x=$\frac{b-a}{11}$mol���ò�Ʒ��Na2CO3����������$\frac{16��b-a��}{11a}$��100%��
�ʴ�Ϊ��$\frac{16��b-a��}{11a}$��100%��
���Ե缫��ⱥ��ʳ��ˮ������2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$2NaOH+H2��+Cl2����������a�缫���������ɵ�NaOH��þ���ӷ�Ӧ��������b�缫���ɺ���B�������Ʒ���������ԭ��Ӧ��
��1�������̿�֪��XΪCl2�����������ԣ��ܽ�����������Ϊ�嵥�ʣ���ӦII�з���������������������ԭ��Ӧ�������ӷ�ӦΪSO2+Br2+2H2O=2HBr+H2SO4��
�ʴ�Ϊ��Cl2��SO2+Br2+2H2O=2HBr+H2SO4��
��2���ó������ⶨ��±��þԪ�صĺ�����g/L����Ӧ֪����Һ�������MgԪ�ص�����������ⶨ��±��Ʒ�������Mg��OH��2������������
�ʴ�Ϊ����±��Ʒ�������Mg��OH��2������������
��3�����200kg ��������Ϊ25%�ı���ʳ��ˮ����Ũ���½���20%ʱ����μӷ�Ӧ��NaClΪ2xmol��
��2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$2NaOH+H2��+Cl2����
2x x x
��$\frac{200000��25%-2x��58.5g/mol}{200000-2g/mol��x-71g/mol��x}$=20%�����x=97.7mol���ռ����������ʵ���Ϊ97.7mol���ʴ�Ϊ��97.7mol��
���� ���⿼����ۺϣ�Ϊ�߿��г����Ŀ��鷽ʽ���漰��ˮ��Դ���á����̷��������������ᴿ�ȣ����ط������ƶϡ������������ۺϿ��飬ע����ԭ�������ӵķŵ�˳������֮��ķ�Ӧ���м��㣬��Ŀ�Ѷ��еȣ�


| A�� | װ��A��ԭ��أ�װ��B�ǵ��� | |
| B�� | ��Ӧһ��ʱ���װ��B����ҺPH���� | |
| C�� | a��������1molCH4��d�ڿɲ���4mol���� | |
| D�� | a��ͨ��C2H6ʱ�ĵ缫��ӦΪ��C2H6-14e-+18OH-�T2CO32-+12H2O |
| ʵ����� | ���� | ʵ����� | |
| �� | ��ij����ͨ��Ʒ����Һ�� | Ʒ����Һ��ɫ | ������һ����SO2 |
| �� | ����ɫ����ͨ����ˮ�� | ��ˮ�Ļ�ɫ��ȥ | �������������ϩ |
| �� | ��ij��Һ�е�������KSCN��Һ | ��Һ���Ժ�ɫ | ����Һһ������Fe2+ |
| ��ȡ����Һ�������ȵ�����ˮ���� ����KSCN��Һ | ��ҺΪ��ɫ | ||
| �� | ��ij��Һ�м��������ữ���Ȼ��� ��Һ | �а�ɫ�������� | ����Һ�п��ܺ���SO42- |
| �� | ��ľ̿��Ũ���ᷴӦ���ɵ�����ͨ�� ����ʯ��ˮ�� | �а�ɫ�������� | ������һ����CO2 |
| A�� | �ڢ� | B�� | �ڢۢ� | C�� | �٢ۢ� | D�� | �٢ڢۢܢ� |
| A | A��һ�ֵ�������Ȼ����Ӳ����� |
| B | BԪ�صĵ�һ�����ܱ�ͬ������������Ԫ�ض��� |
| C | ͬ�����У�CԪ�ص�����������Ӧ��ˮ����ļ�����ǿ |
| D | D�Ļ�̬ԭ��M���������K���2 �� |
| E | E��Cλ�ڲ�ͬ���ڣ�Eԭ�Ӻ���������������C��ͬ�����������Ӿ����� |
��2��A��B��D����Ԫ�ص縺���ɴ�С����˳��ΪN��C��Si
��3��A��B������⻯���зе�ϸߵ�NH3��ԭ����NH3���Ӽ���������
��4����֪��
��AH4��g��+2BO2��g��-B2��g��+AO2��g��+2H2O��g�� H1=-867kJ•mol-1
��2BO2��g��-B2 O4��g�� H2=-56.9kJ•mol-1
д��AH4��B2O4��Ӧ���Ȼ�ѧ����ʽCH4��g��+N2O4��g���TN2��g��+CO2��g��+2H2O��g����H=-810.1kJ/mol��
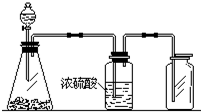 ��ͼ��һ��ʵ��������װ�ã����ڷ�����������ռ����壮���и�������������������װ�ý���ʵ����ǣ�������
��ͼ��һ��ʵ��������װ�ã����ڷ�����������ռ����壮���и�������������������װ�ý���ʵ����ǣ�������| A�� | ̼��ƺ�ϡ���� | B�� | �������̺�Ũ���� | ||
| C�� | п����ϡ���� | D�� | ��ʯ�Һ�Ũ��ˮ |
| A�� | Na2CO3���ܽ��Ա�NaHCO3С | |
| B�� | SiO2���ᡢ����ܷ�Ӧ���������������� | |
| C�� | NO2����ˮʱ����������ԭ��Ӧ | |
| D�� | Fe������Cl2��ȼ������FeCl2��FeCl3 |