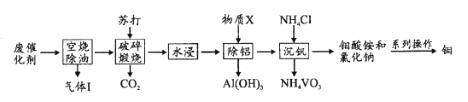
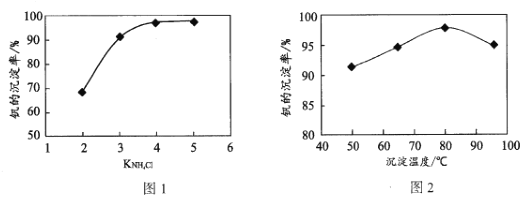
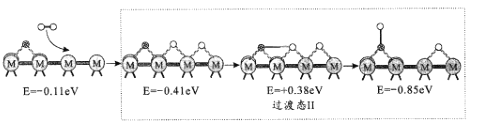
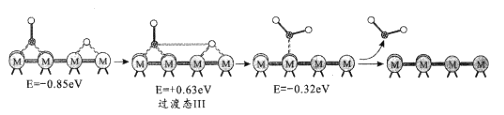
��Ŀ����
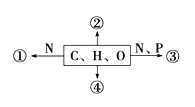
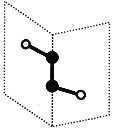
����Ŀ������������Ԫ��X��Y��Z��W��ԭ���������ε���������Y��Zͬ���塣X��Y��Z��W�����γ���ͼ��ʾ�ķ��ӽṹ����Z��W�γɵķ���������ԭ������������8�����ȶ��ṹ������˵������ȷ���ǣ� ��
A.���Ӱ뾶��W>Z>Y>X
B.ZY2��WY2������ʹƷ����Һ��ɫ����ɫԭ����ͬ
C.�������Ӧ��ˮ��������ԣ�W>Z
D.X2Y2��Z2W2�����зǼ��Թ��ۼ��Ҹ�ԭ�Ӷ�����8���ӵ��ȶ��ṹ
���𰸡�B
��������
����������Ԫ��X��Y��Z��W��ԭ���������ε�����X��Y��Z��W�����γ���ͼ��ʾ�ķ��ӽṹ����Z��W�γɵķ���������ԭ������������8�����ȶ��ṹ�����ͼʾ��ZΪS��WΪClԪ�أ�����Y��Zͬ���壬��YΪOԪ�أ�X��Y�γɵĸû�����Ϊ˫��ˮ����XΪHԪ�ء�
���ݷ�����֪��XΪH��YΪO��ZΪS��WΪClԪ�ء�
A��ͬһ���ڴ������������Ӱ뾶��С��S2��>Cl����ͬһ������ϵ��������Ӱ뾶������S2��>O2������A����
B��SO2��ClO2������ʹƷ����Һ��ɫ����ɫԭ����ͬ��ǰ�����ɲ��ȶ�����ɫ���ʣ����߷���������ԭ��Ӧ����B��ȷ��
C��Ӧ������������Ӧ��ˮ��������ԣ�W>Z����C����
D��H2O2��H������8���ӵ��ȶ��ṹ����D����
��ѡB��

 ����һ������ܼƻ�ϵ�д�
����һ������ܼƻ�ϵ�д�