ÌâÄżÄÚÈĘ
ĄŸÌâÄżĄżCOșÍH2żÉŚśÎȘÄÜÔŽșÍ»Żč€ÔÁÏŁŹÓŠÓĂÊź·Öčă·șĄŁč€Ò”ÉÏżÉÀûÓĂCO»òCO2ÓëH2·ŽÓŠÀŽÖƱžŒŚŽŒĄŁ
·ŽÓŠąÙŁș2H2(g)+CO(g)![]() CH3OH(g)ĄśHŁœŁ90.8kJĄ€mol-1
CH3OH(g)ĄśHŁœŁ90.8kJĄ€mol-1
·ŽÓŠąÚŁșH2(g)+CO2(g)![]() H2O(g)+CO(g)ĄśHŁœ+41.2kJĄ€mol-1
H2O(g)+CO(g)ĄśHŁœ+41.2kJĄ€mol-1
Łš1Ł©ĐŽłöÓĂCO2ÓëH2·ŽÓŠÖƱžŒŚŽŒ”ÄÈÈ»ŻŃ§·œłÌÊœ______________ĄŁ
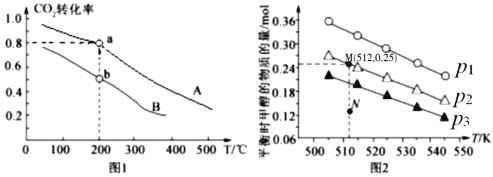
Łš2Ł©ÒŃÖȘ”ÈÌć»ę”ÄÒ»Ńő»ŻÌŒșÍËźŐôÆűœűÈë·ŽÓŠÆśÊ±ŁŹ»á·ąÉúÈçÏ·ŽÓŠŁșCO(g)+H2O(g)![]() H2(g)+CO2(g)ŁŹžĂ·ŽÓŠÆœșⳣÊęËæÎÂ¶È”Ä±ä»ŻÈç±íËùÊŸŁș
H2(g)+CO2(g)ŁŹžĂ·ŽÓŠÆœșⳣÊęËæÎÂ¶È”Ä±ä»ŻÈç±íËùÊŸŁș
ζÈ/Ąæ | 400 | 500 | 800 |
ÆœșⳣÊęK | 9.94 | 9 | 1 |
ÉęžßÎÂ¶ÈŁŹžĂÆœșâ”ÄÒƶŻ·œÏòÊÇ______________ŁšÌŐęÏòĄ±»òĄ°ÄæÏòĄ±Ł©ĄŁ500ĄæʱŁŹCOșÍH2O”ÄÆđÊŒĆš¶ÈŸùÎȘ0.020molĄ€L-1ŁŹžĂÌőŒțÏÂCO”ÄÆœșâĆš¶ÈÎȘŁș______________molĄ€L-1ĄŁ
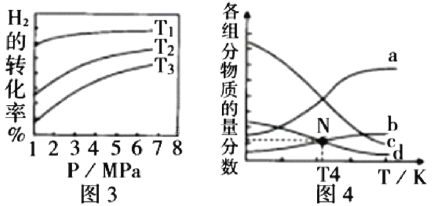
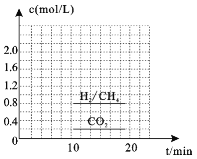
Łš3Ł©Ò»¶šÌőŒțÏÂŁŹżÉÒÔÓÉCO2(g)șÍH2(g)șÏłÉCH4(g)ŁŹÍŹÊ±»čÉúłÉH2O(g)ĄŁÏòșăÈĘĂܱŐÈĘÆśÖĐłäÈëÒ»¶šÁż”ÄCO2șÍH2ŁŹÔÚ300Ąæʱ·ąÉúÉÏÊö·ŽÓŠŁŹ10minŽï”œÆœșâÊ±Čż·ÖÎïÖÊ”ÄÁżĆš¶ÈÈçÍŒËùÊŸ,žĂζÈÏ”ĔÄÆœșⳣÊę”ÈÓÚ_______________ĄŁ
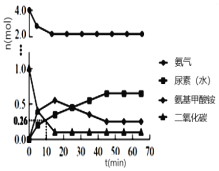
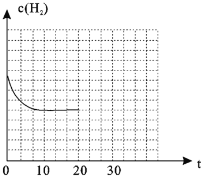
Łš4Ł©ÈôÔÚ20minʱŒőĐĄŃčÇżŁŹČąÔÚ30minʱŽï”œÆœșâŚŽÌŹŁŹÇëÔÚÍŒ2ÖĐ»łöH2”ÄÎïÖÊ”ÄÁżĆš¶ÈËæʱŒä±ä»Ż”ÄÍŒÏń__________________ĄŁ
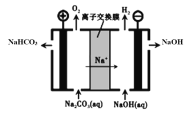
Łš5Ł©č€Ò”ÉÏ”çœâNa2CO3ÈÜÒșżÉÒÔÉúłÉNaHCO3șÍNaOHÁœÖÖč€Ò”ÖŰÒȘÔÁÏŁŹŚ°ÖĂÈçÍŒËùÊŸĄŁÇëĐŽłöŃôŒ«”Ĕ猫·ŽÓŠÊœ______________________ĄŁ
ĄŸŽđ°žĄżCO2(g)+3H2(g)ŁœCH3OH(g)+H2O(g)ĄśH=-49.6kJĄ€mol-1 ÄæÏò 0.005 25  4CO32-+2H2O-4e-Łœ4HCO3-+O2Ąü
4CO32-+2H2O-4e-Łœ4HCO3-+O2Ąü
ĄŸœâÎöĄż
Łš1Ł©ÒŃÖȘŁș·ŽÓŠąÙŁș2H2(g)+CO(g)![]() CH3OH(g)ĄśHŁœŁ90.8kJĄ€mol-1
CH3OH(g)ĄśHŁœŁ90.8kJĄ€mol-1
·ŽÓŠąÚŁșH2(g)+CO2(g)![]() H2O(g)+CO(g)ĄśHŁœ+41.2kJĄ€mol-1
H2O(g)+CO(g)ĄśHŁœ+41.2kJĄ€mol-1
ÓÉžÇË趚ÂÉżÉÖȘąÙ+ąÚżÉ”ĂCO2(g)+3H2(g)ŁœCH3OH(g)+H2O(g)ĄśH=-49.6kJĄ€mol-1ŁŹčÊŽđ°žÎȘŁșCO2(g)+3H2(g)ŁœCH3OH(g)+H2O(g)ĄśH=-49.6kJĄ€mol-1Ł»
Łš2Ł©Î¶ÈÉęžßKÖ”ŒőĐĄŁŹÔòÆœșâÏòÄæ·œÏòÒƶŻŁ»ÉèCO”ÄĆš¶È±ä»ŻÎȘxmol/LÔò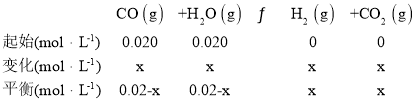
![]() Ôòx=0.015mol/LŁŹÔòCO”ÄÆœșâĆš¶ÈÎȘ0.02 mol/L -0.015 mol/L=0.005 mol/LŁŹčÊŽđ°žÎȘŁșÄæÏòŁ»0.005Ł»
Ôòx=0.015mol/LŁŹÔòCO”ÄÆœșâĆš¶ÈÎȘ0.02 mol/L -0.015 mol/L=0.005 mol/LŁŹčÊŽđ°žÎȘŁșÄæÏòŁ»0.005Ł»
Łš3Ł©Ò»¶šÌőŒțÏÂŁŹżÉÒÔÓÉCO2(g)șÍH2(g)șÏłÉCH4(g)ŁŹÍŹÊ±»čÉúłÉH2O(g)·ŽÓŠÎȘŁș![]() ŁŹžùŸĘÍŒÏńżÉÖȘc(CH4)=0.8mol/LŁŹÔòc(H2O)= 2c(CH4)=1.6mol/LŁŹ
ŁŹžùŸĘÍŒÏńżÉÖȘc(CH4)=0.8mol/LŁŹÔòc(H2O)= 2c(CH4)=1.6mol/LŁŹ  ŁŹčÊŽđ°žÎȘŁș25Ł»
ŁŹčÊŽđ°žÎȘŁș25Ł»
Łš4Ł©![]() ŒőĐĄŃčÇżŁŹÈĘÆśÌć»ęÔöŽóŁŹĆš¶ÈŒőĐĄŁŹÆœșâÄæÒÆŁŹžùŸĘÀŐÏÄÌŰÁĐÔÀíżÉÖȘŚîÖŐĆš¶ÈĐĄÓÚÆœșâ10minÖĐÊ±Ćš¶ÈŁŹÍŒÏńÎȘ
ŒőĐĄŃčÇżŁŹÈĘÆśÌć»ęÔöŽóŁŹĆš¶ÈŒőĐĄŁŹÆœșâÄæÒÆŁŹžùŸĘÀŐÏÄÌŰÁĐÔÀíżÉÖȘŚîÖŐĆš¶ÈĐĄÓÚÆœșâ10minÖĐÊ±Ćš¶ÈŁŹÍŒÏńÎȘ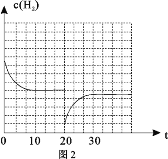 ŁŹčÊŽđ°žÎȘŁș
ŁŹčÊŽđ°žÎȘŁș Ł»
Ł»
Łš5Ł©ÓÉ”çœâŚ°ÖĂÊŸÒâÍŒżÉÖȘŁŹșÍ”çÔŽŐęŒ«ÏàÁŹ”ÄÎȘŃôŒ«ŁŹ”çœâÖÊÎȘNa2CO3ÈÜÒșÉúłÉO2șÍNaHCO3ŁŹŃôŒ«”猫·ŽÓŠÎȘŁș4CO32-+2H2O-4e-Łœ4HCO3-+O2ĄüŁŹčÊŽđ°žÎȘŁș4CO32-+2H2O-4e-Łœ4HCO3-+

ĄŸÌâÄżĄżÊ”ŃéÊÒÖƱžŽżŒî”ÄÖśÒȘČœÖèÈçÁśłÌËùÊŸŁș

ËÄÖÖŃÎÔÚȻ͏ζÈÏ”ÄÈÜœâ¶ÈŁšg/100gH2OŁ©±í
ÎÂ¶È ÎïÖÊ | 0Ąæ | 10Ąæ | 20Ąæ | 30Ąæ | 40Ąæ | 50Ąæ | 60Ąæ | 100Ąæ |
NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 39.8 |
NH4HCO3 | 11.9 | 15.8 | 21.0 | 27.0 | - | - | - | - |
NaHCO3 | 6.9 | 8.1 | 9.6 | 11.1 | 12.7 | 14.5 | 16.4 | - |
NH4Cl | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.3 | 77.3 |
ÌáÊŸŁșζȞßÓÚ35ĄæʱNH4HCO3»á·ÖœâŁŹÇë»ŰŽđŁș
Łš1Ł©ÏÂÁĐČÙŚś»òĂèÊöŐęÈ·”ÄÊÇ________ĄŁ
AŁźÎÂ¶ÈżŰÖÆÔÚ30-35ĄæÊÇÒòÎȘζÈÌ«žßNH4HCO3»á·ÖœâŁŹÎ¶ÈÌ«”Í·ŽÓŠËÙÂÊÌ«Âę
BŁź±ŁÎÂ30min”ÄÄż”ÄÊÇÊč·ŽÓŠłä·ÖœűĐĐ
CŁźčęÂËșó”ÄÂËÒșÖ»ÓĐNH4ClșÍNH4HCO3ÈÜÖÊ
DŁźÏŽÈ„Ÿ§Ìć±íĂæ”ÄÔÓÖÊżÉÒÔŃĄÓĂŐôÁóËź
Łš2Ł©·ŽÓŠÎÂ¶ÈżŰÖÆÔÚ30Ą«35ĄæŁŹÎȘżŰÖÆŽËζȷ¶Î§ŁŹČÉÈĄ”ÄŒÓÈÈ·œ·šÎȘ______________ĄŁ
Łš3Ł©łŁÎÂʱŁŹčęÂËșóÖśÒȘ”Ă”œNaHCO3Ÿ§Ìć”ÄÔÒòÊÇ______________ĄŁ
Łš4Ł©ŚÆÉŐNaHCO3Ÿ§Ìć”ÄŚ°ÖĂÎȘ________ĄŁ
A.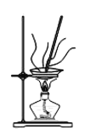 B.
B.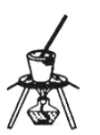 C.
C.
Łš5Ł©ÏŽ”ÓNaHCO3Ÿ§Ìć”ÄČÙŚś______________ĄŁ
Łš6Ł©Č⶚ŽżŒîČúÆ·ÖĐNaHCO3șŹÁż”Ä·œ·šŁșŚŒÈ·łÆÈĄŽżŒîŃùÆ·Wg·ĆÈ댶ĐÎÆżÖĐŒÓŐôÁóËźÈÜœâŁŹŒÓ1Ą«2”ηÓÌȘ֞ʟŒÁŁŹÓĂÎïÖÊ”ÄÁżĆš¶ÈÎȘcŁšmolĄ€L-1Ł©”ÄHClÈÜÒș”ζšÖÁÈÜÒșÓÉșìÉ«”œÎȚÉ«(֞ʟCO32-+H+=HCO3Ł·ŽÓŠ”ÄÖŐ”ă)ËùÓĂHClÈÜÒșÌć»ęÎȘV1mLĄŁÔÙŒÓ1Ą«2”ÎŒŚ»ùłÈ֞ʟŒÁŁŹŒÌĐűÓĂHClÈÜÒș”ζšÖÁÈÜÒșÓɻƱäłÈŁŹËùÓĂHClÈÜÒșÌć»ęÎȘV2mLŁŹĐŽłöŽżŒîŃùÆ·ÖĐNaHCO3ÖÊÁż·ÖÊę”ÄŒÆËăÊœŁș______________ĄŁ