��Ŀ����
����Ŀ��ͭ���ѡ��ܼ��仯����������������Ҫ���ã��ش���������
(1)��Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ__________��δ�ɶԵ�����Ϊ________________��
(2)Ti(BH4)2��һ�ֹ���Ԫ�����⻯�ﴢ����ϡ�
����BH4-��Ϊ�ȵ�����������ӵĻ�ѧʽΪ_____��
��H��B��Tiԭ�ӵĵ�һ��������С�����˳��Ϊ_____��
(3)��������(TiO2)�dz��õġ����нϸߴ����Ժ��ȶ��ԵĹ��������������ˮ����������TiO2����һ��ʵ����ͼ��ʾ��
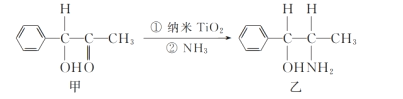
�������ҵķ����в�ȡsp3�ӻ���ʽ��ԭ�Ӹ���Ϊ_____��
(4)�����[Co(NH3)4(H2O)2]Cl3������Ҫ������
[Co(NH3)4(H2O)2]Cl3�е�Co3+��λ��Ϊ___�������ӵ����幹����________________��
(5)ͭ����±��(SCN)2��Ӧ����Cu(SCN)2��1 mol(SCN)2�к�����������ĿΪ_____��HSCN�ṹ�����֣���֪������(H��S��C��N)�ķе������������(H��N=C=S)����ԭ����_______��
���𰸡�1s22s22p63s23p63d74s2��[Ar]3d74s2 3 NH4+ Ti<B<H 5 6 ������ 4NA ��������(H-N=C=S)������Nԭ����������Hԭ�ӣ����Ӽ����γ����
��������
(1)��Ԫ��Ϊ��27��Ԫ�أ����ݹ���ԭ����д��̬ԭ�Ӻ���ĵ����Ų�ʽ��
(2)��BH4-����5��ԭ�ӣ��۵�������Ϊ8���ݴ˷�����д��BH4-��Ϊ�ȵ�����������ӣ�
�ڸ��ݵ�һ�����ܵı仯���ɷ����ж�H��B��Ti�ĵ�һ�����ܴ�С��
(3)��ȡsp3�ӻ���ʽ��ԭ�ӵļ۲���Ӷ���=4���ݴ˷����жϷ����в�ȡsp3�ӻ���ʽ��ԭ�Ӹ�����
(4) �������[Co(NH3)4(H2O)2]Cl3�У�����ΪNH3��H2O�����ݼ۵��ӻ��������ж������ӵ����幹�ͣ�
(5) (SCN)2���ӽṹʽΪN��C-S-S-C��N��1��N��C������1����������������Ϊ��������������(H-N=C=S)������Nԭ����������Hԭ�ӣ����Ӽ����γ������
(1)��Ԫ��Ϊ��27��Ԫ�أ����ݹ���ԭ�������̬ԭ�Ӻ���ĵ����Ų�ʽΪ��1s22s22p63s23p63d74s2��[Ar]3d74s2�����ݺ��ع���������ͬ�����Ĺ���Ų�ʱ��������ռ�ݲ�ͬ�����������3d�������3��δ�ɶԵ��ӣ���δ�ɶԵ�����Ϊ3��
(2)��BH4-����5��ԭ�ӣ��۵�������Ϊ8��������BH4-��Ϊ�ȵ��������������NH4+��
��Ti�ǽ���Ԫ�أ�Ti�ĵ�һ��������С��H��Bʧ��������B����H�����Ե�һ�����ܵĴ�С˳����Ti<B<H��
(3)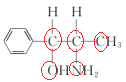 �л���Ȧ��ԭ�Ӽ۲���Ӷ���=4�����Բ�ȡsp3�ӻ���ʽ��ԭ�Ӹ���Ϊ5��
�л���Ȧ��ԭ�Ӽ۲���Ӷ���=4�����Բ�ȡsp3�ӻ���ʽ��ԭ�Ӹ���Ϊ5��
(4) �������[Co(NH3)4(H2O)2]Cl3�У�����ΪNH3��H2O������λ��=4+2=6���������ӵijɼ����Ӷ���Ϊ6���µ��Ӷ���Ϊ0�������幹��Ϊ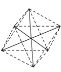 ���ǰ����壻
���ǰ����壻
(5) (SCN)2���ӽṹʽΪN��C-S-S-C��N��1��N��C������1����������������Ϊ������1mol(SCN)2�����к�����������ĿΪ4NA�����������������ķ���ʽ��ͬ���ṹ��ͬ����Ϊͬ���칹�壬��������(H-N=C=S)������Nԭ����������Hԭ�ӣ����Ӽ����γ�������ʷе�ߣ�