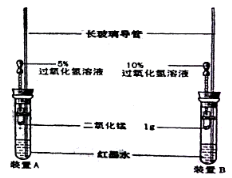
��Ŀ����
����Ŀ����NA��ʾ�����ӵ�����������˵����ȷ���ǣ� ��
���ڱ�״���£�NA��SO3������ռ�����Ϊ22.4L
��S2��S8�Ļ���ﹲ6.4g������������ԭ����һ��Ϊ0.2NA
����״ף�£�22.4LNO��11.2LO2��Ϻ�����ķ�������Ϊ1.5 NA
��1molAlCl3����1L��ˮ�У�������Һ����1NAAl3+
���ڱ�״���£�22.4LCl2������������������Һ��Ӧת�Ƶĵ�����ΪNA
��100mL1mol/LFe2(SO4)3��Һ�У�Fe3+��SO42-���������ܺ���0.5NA
A������ B���������� C�������� D����������
���𰸡�A
��������
������������ڱ�״����SO3������̬��������������Ħ��������������������S2��S8�Ļ���ﹲ6.4g������������ԭ����һ��Ϊ![]() ��0.2NA����ȷ������״ף�£�22.4LNO��11.2LO2��Ϻ�ǡ������22.4LNO2����NO2����ƽ��2NO2
��0.2NA����ȷ������״ף�£�22.4LNO��11.2LO2��Ϻ�ǡ������22.4LNO2����NO2����ƽ��2NO2![]() N2O4��������ķ�������С��NA��������1molAlCl3����1L��ˮ�У�����������ˮ�⣬���������Һ���е�Al3+С��1mol���������ڱ�״���£�22.4LCl2������������������Һ��Ӧת�Ƶĵ�����ΪNA����ȷ����100mL1mol/LFe2(SO4)3��Һ��������ˮ�⣬ע��Fe3+��SO42-���������ܺ�С��0.5NA������ѡA��
N2O4��������ķ�������С��NA��������1molAlCl3����1L��ˮ�У�����������ˮ�⣬���������Һ���е�Al3+С��1mol���������ڱ�״���£�22.4LCl2������������������Һ��Ӧת�Ƶĵ�����ΪNA����ȷ����100mL1mol/LFe2(SO4)3��Һ��������ˮ�⣬ע��Fe3+��SO42-���������ܺ�С��0.5NA������ѡA��

 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�����Ŀ��W��X��Y��Z���ֶ�����Ԫ�ص�ԭ��������X��W��Z��Y��Wԭ�ӵ������û��p���ӣ�Xԭ�Ӻ���s��������p������֮��Ϊ1��1��Yԭ�������s������֮��Ϊ1��1��Zԭ�ӵ�p��������Yԭ�ӵĶ�2����
��1������Ԫ��ԭ�Ӱ뾶�Ӵ�С��˳��Ϊ ����Ԫ�ط��ű�ʾ����
��2����������������������գ�
��һ������ | �縺�� | �⻯��е� | ����������Ӧˮ����ļ��� |
W������������X | Z������������Y | Y������������Z | W������������X |
��3��д��XԪ�صĵ�����Z��Y���γɵĻ����ﷴӦ�Ļ�ѧ����ʽ�����������ת�Ƶķ������Ŀ�� ��
����Ŀ��һ��ԭ��ص��ܷ�Ӧ�����ӷ���ʽ��Zn��Cu2��===Zn2����Cu����ԭ��صĺ������
���� | ���� | �������Һ | |
A | Zn | Cu | CuCl2 |
B | Cu | Zn | H2SO4 |
C | Cu | Zn | CuSO4 |
D | Zn | Fe | CuCl2 |
����Ŀ����1����O2��HC1ת��ΪCl2�������Ч�棬������Ⱦ����ͳ�ϸ�ת��ͨ������ͼ��ʾ�Ĵ���ѭ��ʵ�֣�

���У���Ӧ��Ϊ��2HCl(g)+CuO(s) ![]() H2O(g)+CuCl2��s�� ��H1����Ӧ������1 mol Cl2 (g)�ķ�Ӧ��Ϊ��H2�����ܷ�Ӧ���Ȼ�ѧ����ʽΪ____________________________������Ӧ������H1����H2��ʾ����
H2O(g)+CuCl2��s�� ��H1����Ӧ������1 mol Cl2 (g)�ķ�Ӧ��Ϊ��H2�����ܷ�Ӧ���Ȼ�ѧ����ʽΪ____________________________������Ӧ������H1����H2��ʾ����
��2��һ�������²��������Ӧ������c(Cl2)���������£�
t��min�� | 0 | 2.0 | 4.0 | 6.0 | 8.0 |
c(Cl2)/10-3(mol/L) | 0 | 1.8 | 3.7 | 5.4 | 7.2 |
����2.0��6.0min����HCl�����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ���� _________��
��3�������£�����ȥ��������Ĥ��Al��CuƬ����ŨHNO3�����ԭ��أ�ͼ1�������ԭ��صĵ���ǿ�ȣ�I����ʱ�䣨t���ı仯��ͼ2��ʾ����Ӧ�������к���ɫ���������

0��tlʱ��ԭ��صĸ�����AlƬ����ʱ�������ĵ缫��Ӧʽ��______________����Һ�е�H+��______���ƶ���tlʱ��ԭ����е��������������ı䣬��ԭ����_____________��