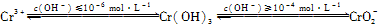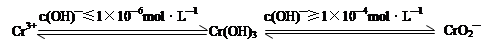��Ŀ����
��ij������Һ�У���Cl-֮����ܻ����е����ʵ����������������е�һ�ֻ���֣�SO32-��CO32-��SiO32-��I-��NO3-��SO42-���ڴ���Һ�м���������ᣬ�������ݣ���Һ��ɫ������Գ��壬����ԭ��Һ�е����������������3�֡��Իش��������⡣
��1��ԭ��Һ���Ƿ���SiO32-�� ����С���û�С������ж������� ���������ӷ���ʽ��ʾ��
��2�����ɵ�������һ���� �������е������� (����ĸ���)��
| A����ɫ��ζ |
| B����ɫ�д̼�����ζ |
| C�����ڴ�����Ⱦ�� |
| D��������ˮ |
��3��ԭ��Һ�п��ܺ��е��������� ��
��4����Һ�м��ٵ������� ��ԭ���� �������ӷ���ʽ��ʾ����
��1��û�У�1�֣���SiO32-+2H+=H2SiO3����1�֣�
��2��NO��1�֣���ACD��1�֣���
��3��SO42-��1�֣�
��4��SO32-��I-��NO3-����2�֣�
6I-+8H++2NO3-=3I2+2NO��+4H2O����2�֣�
3SO32-+2H++2NO3-=3SO42-+2NO��+H2O��2�֣�
��������������������������Һ�Գ����֪��SiO32����SiO32-+2H+=H2SiO3������Һ��ɫ���һ����I�� NO3��������Ӧ��6I����2NO3����8H��=3I2��2NO����4H2O�����������һ����NO�������������ʵ�������ȣ�����������Ӧ��NO3����ʣ�࣬��ˣ�ʹNO3��ȫ����Ӧ����Ҫ��SO32����3SO32-+2H++2NO3-=3SO42-+2NO��+H2O�������ʵ�����SO32����I�� ��NO3��ǡ����ȫ��Ӧ2I����2SO32-��2NO3����4H��=3I2��2NO����2SO42-��2H2O������3�����Ӽ��٣������CO32������Һ�м��ٵ�����Ӧ��SO32����I�� ��NO3����ԭ��Һ�п��ܺ��е���������SO42-��
���㣺�������Ӽ���Ͷ��������ƶϣ����ѡ�

 һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�ij��ɫ��Һ�������п��ܴ����������ӣ�Na+��Ag+��Ba2+��Al3+��AlO2-��S2-��CO32-��SO32-��SO42-����ȡ����Һ�����й����飬�������£�
�ش��������⣺
��1�����ɳ��������ӷ���ʽ�� ��
��֪�������������������ɣ�������ˮ������HBr�����������ʵ����һ���������ֳɷ֣��������Լ��Լ����������±��У��ɲ�����������ÿһ�ж�Ӧ��ȷ���ɵ÷֣�
| ���� | ʵ��Ŀ�� | �Լ� | ���� |
| 1 | | | |
| 2 | | | |
| 3 | | | |
| 4 | | | |
��2������Һ�����ɳ����ҵ����ӷ���ʽ�� ��
��3����������������Һ�п϶����ڵ������У� ��
��4�������Һ�п��ܴ��ڵ����ӣ���ʵ����֤���Ƿ���ڵķ�����
��
����Fe2����I����Br������Һ��ͨ��������������Һ�и������ӵ����ʵ����仯��ͼ��ʾ���й�˵������ȷ����( )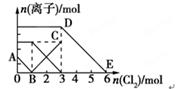
| A���߶�BC����Fe3�����ʵ����ı仯��� |
| B��ԭ�����Һ��n(FeBr2)=3mol |
| C����ͨ��2molCl2ʱ����Һ���ѷ��������ӷ�ӦΪ��2Fe2��+2I��+2Cl2=2Fe3��+I2+4Cl�� |
| D��ԭ��Һ��n(Fe2��)��n(I��)��n(Br��)=2��1��3 |