��Ŀ����
�����������ж���������Σ���ܴ���˺�����ˮ������д��������ŷš�
��֪��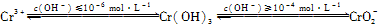
��1���ں���6�۸��ķ�ˮ�м���һ���������������������ʹ��6�۸���ԭ�ɣ�3�۸����ٵ�����ҺpH��6��8֮�䣬ʹFe3����Cr3��ת��ΪFe(OH)3��Cr(OH)3��������ȥ��
��д��Cr2O72-��FeSO4��Һ�����������·�Ӧ�����ӷ���ʽ��________________��
�������ӷ���ʽ��ʾ��ҺpH���ܳ���10��ԭ��____��
��2��������6�۸��ķ�ˮ��������ڣ������������������������Ȼ��ƽ��е�⡣���������ɵ�Fe2����Cr2O72-������Ӧ�����ɵ�Fe3����Cr3������������OH����ϳ����������������ȥ��
��д�������ĵ缫��Ӧʽ��________________��
�ڵ�ⷨ�м����Ȼ��Ƶ�������________________��
��3������ѧ�ḻ��ʣ����ڸ�����Ⱥã����������������������棬ͬ��������ɸ������ܵIJ���֣�CrO3���������ڵ�ƹ�ҵ�С�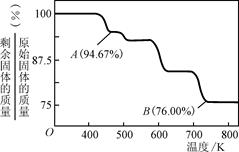
��CrO3����ǿ�����ԣ������л���(��ƾ�)ʱ�����ҷ�Ӧ�����Ż����ù������Ҵ������������ᣬCrO3����ԭ����ɫ�������[Cr2(SO4)3]����÷�Ӧ�Ļ�ѧ����ʽΪ____��
��CrO3�����ȶ��Խϲ����ʱ�ֽ⣬�������������¶ȵı仯����ͼ��ʾ����B��ʱʣ�����ijɷ���________________(�ѧʽ)��
��1����Cr2O72-��6Fe2����14H��=2Cr3����6Fe3����7H2O
��Cr(OH)3��OH��=CrO2-��2H2O
��2����2H����2e��=H2��(��2H2O��2e��=H2����2OH��)
����ǿ��Һ������
��3����4CrO3��3C2H5OH��6H2SO4=2Cr2(SO4)3��3CH3COOH��9H2O
��Cr2O3
�����������������1�������������л�ԭ�ԣ�Cr2O72-��ǿ�����ԣ������ܷ���������ԭ��Ӧ�����������ӱ����������������ӣ�Cr2O72-����ԭΪCr3+����Ӧ����ʽΪ��Cr2O72-+6Fe2++14H+�T2 Cr3++6Fe3++7H2O���ʴ�Ϊ��Cr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O�����������֪����pH����10��c��OH-����10-4mol?L-1ʱ��Cr��OH��3ת���CrO2-��Cr��OH��3+OH-�TCrO2-+2H2O���ʴ�Ϊ��Cr��OH��3+OH-�TCrO2-+2H2O����2���ٽ���+6�۸��ķ�ˮ��������ڣ������������������������Ȼ��ƽ��е�⣬��������ʧ�������ɶ��������ӣ������������ӵõ��������������缫��ӦʽΪ��2H++2e-�TH2����2H2O+2e-�TH2��+2OH-���ʴ�Ϊ��2H++2e-�TH2����2H2O+2e-�TH2��+2OH-����ˮ��������ʣ���������ˮ�ĵ���������С���Ȼ�����ǿ����ʣ���ˮ������ȫ���뵼����Һ��������Ũ��������������ǿ��Һ�����ԣ��ʴ�Ϊ����ǿ��Һ�����ԣ���3����CrO3����ǿ�����ԣ������л����ƾ���ʱ���Ҵ������������ᣬ̼��ƽ�����ϼ۴�-2�����ߵ�0��1���Ҵ����ϼ۱仯4��CrO3����ԭ����ɫ�������[Cr2��SO4��3]�����Ļ��ϼ۴�+6�۽��͵�+3�ۣ�1��CrO3���ϼ۱仯3�����ߵ���С��������12���ٸ���ԭ���غ�ã�4CrO3+3C2H5OH+6H2SO4=2Cr2��SO4��3+3CH3CO0H+9H2O���ʴ�Ϊ��4CrO3+3C2H5OH+6H2SO4=2Cr2��SO4��3+3CH3CO0H+9H2O������CrO3������Ϊ100g����CrO3�и�Ԫ�ص�����Ϊ��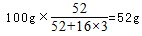 ����B��ʱ���������Ϊ��100g��76%=76g��Co������û�б䣬������������Co������Ϊ52g����Ԫ�ص�����Ϊ16�����ߵĸ�����Ϊ
����B��ʱ���������Ϊ��100g��76%=76g��Co������û�б䣬������������Co������Ϊ52g����Ԫ�ص�����Ϊ16�����ߵĸ�����Ϊ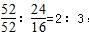 ������B��ʱʣ�����ijɷ���Cr2O3���ʴ�Ϊ��Cr2O3��
������B��ʱʣ�����ijɷ���Cr2O3���ʴ�Ϊ��Cr2O3��
���㣺���⿼�������ӷ���ʽ�͵缫��Ӧʽ����д���ѶȲ���ע�⣨3�������У��ڱ仯�����У�Co������û�б䣬�ǽ���Ĺؼ���

������A��B����ѧ���������ʣ����������ӿɴ��±���ѡ��
| ������ | K����Na����Fe2����Ba2����NH4+ |
| ������ | OH����NO3����I����HCO3����AlO2����HSO4�� |
(1)��A��B��ˮ��Һ��Ϊ��ɫ��B��ˮ��Һ�ʼ��ԣ��һ�Ϻ�ֻ����������ϡ����İ�ɫ��������ʹ��ɫʯ����ֽ���������塣
��B�Ļ�ѧʽΪ__________________��
��A��B��Һ��Ϻ���ȳ����ԣ���Ӧ�����ӷ���ʽΪ__________________________��
(2)��A��ˮ��Һ��dz��ɫ��B��ˮ��Һ��ɫ������ɫ��ӦΪ��ɫ����A��ˮ��Һ�м���ϡ���������������ټ���B����Һ��ƣ���A��B��ˮ��Һ����������Ա仯����
��AΪ_______________��
�ھ�����������������Һ��Ƶ�ԭ����������֣�
��._______ _____________________�� ��.___________ ____________��
������һ������֤��������Һ��Ƶ�ԭ��__________________________________��
��������Һ���ԭ����������Ƴ�ԭ��أ���������a����b����b���ĵ缫��ӦʽΪ_______________________________________
I��NaOH��FeCl3����ѧ��ѧʵ���ҳ��õ��Լ���
��1����һ������������NaOH��Һ��Ӧ�Ĺ��嵥����________��________������������
����һ�����ʵ���Ũ�ȵ�NaOH��Һʱ�����ʵ����ƫ�͵�ԭ����________��
| A������ƿ��ԭ����������ˮ |
| B��ϴ���ձ��Ͳ���������Һδת������ƿ�У� |
| C������ʱ�۲�Һ�温�� |
| D���ܽ��δ����ȴ����ת��������ƿ�� |
______________________________________________.
�������ȼ��ʱ�ܷ���������ȣ���Ҳ��Һ��ʯ��������Ҫ�ɷ֣���Ϊ��ԴӦ��������
���ճ����������
��֪��
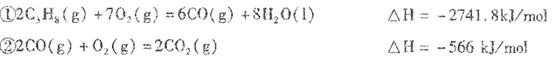
��1����Ӧ
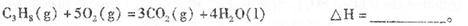
��2�����ݣ�1���еķ�Ӧ�������һ������ȼ�ϵ�أ�һ��ͨ�˿�������һ��ͨ��������壺ȼ�ϵ���ڲ������ڵIJ�����������(Y2O3)�������(ZrO2)���壬�����ڲ����Դ���O2�����ڵ���ڲ�O2������_________�������������������صĸ�����ӦΪ_____________________________.
��3��������ȼ�ϵ���ö��Ե缫�������Mg(NO3)2��NaCl�Ļ����Һ����ʼ������������Ϊ_______________________________________________��
��ij������Һ�У���Cl-֮����ܻ����е����ʵ����������������е�һ�ֻ���֣�SO32-��CO32-��SiO32-��I-��NO3-��SO42-���ڴ���Һ�м���������ᣬ�������ݣ���Һ��ɫ������Գ��壬����ԭ��Һ�е����������������3�֡��Իش��������⡣
��1��ԭ��Һ���Ƿ���SiO32-�� ����С���û�С������ж������� ���������ӷ���ʽ��ʾ��
��2�����ɵ�������һ���� �������е������� (����ĸ���)��
| A����ɫ��ζ |
| B����ɫ�д̼�����ζ |
| C�����ڴ�����Ⱦ�� |
| D��������ˮ |
��3��ԭ��Һ�п��ܺ��е��������� ��
��4����Һ�м��ٵ������� ��ԭ���� �������ӷ���ʽ��ʾ����
 ��
�� ��Ϊ�˽�һ��ȷ�ϣ�ȡ������ʵ���⣺
��Ϊ�˽�һ��ȷ�ϣ�ȡ������ʵ���⣺