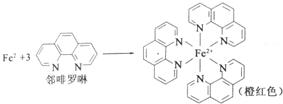
��Ŀ����
16������ͭ��ȡ��M��ͨ����ͼ����ʾ��Ӧʵ��ͭ���ӵĸ�������1��X������ˮ���������л��ܼ����侧������Ϊ���Ӿ��壮
��2��M����Ԫ�صĵ縺���ɴ�С˳��ΪO��N��C��H��������̼ԭ�ӵ��ӻ���ʽ��sp2��sp3��
��3��������Ӧ�ж��Ѻ����ɵĻ�ѧ����be������ţ���
a�����Ӽ���b����λ����c����������d�����»�����e�����ۼ�
��4��M��W�����ӽṹ��ͼ�ң���ȣ�M��ˮ����С��������Cu2+����ȡ��Mˮ����С����Ҫԭ����M���γɷ����������ʹ�ܽ�ȼ�С��
��5����̬Cu2+����Χ�����Ų�ʽΪ3d9��Cu2+�ȹ���Ԫ��ˮ�������Ƿ�����ɫ��ԭ�ӽṹ�йأ��Ҵ���һ���Ĺ��ɣ��ж�Sc3+��Zn2+��ˮ������Ϊ��ɫ��������3d�����û��δ�ɶԵ��ӣ�3d����ϵ���Ϊȫ�ջ�ȫ������
| ���� | Sc3+ | Ti3+ | Fe2+ | Cu2+ | Zn2+ |
| ��ɫ | ��ɫ | �Ϻ�ɫ | dz��ɫ | ��ɫ | ��ɫ |
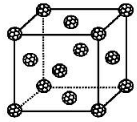
�پ���Ļ�ѧʽ��CuCl��
����֪�þ�����ܶ�Ϊ��g•cm-3������٤��������ֵΪNA����þ�����ͭԭ����Yԭ��֮�����̾���Ϊ=$\frac{\sqrt{3}}{4}\root{3}{\frac{4��99.5}{��{N}_{A}}}$��1010pm��ֻд����ʽ����
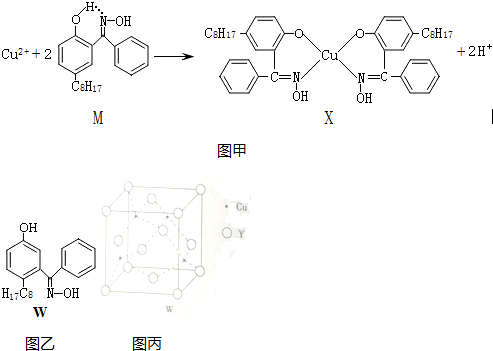
���� ��1���л���Ĺ��������Ƿ��ӣ��ɷ��ӹ��ɵľ����Ƿ��Ӿ��壻
��2��M�к���C��H��O��NԪ�أ�Ԫ�صķǽ�����Խǿ����縺��Խǿ�����ݼ۲���ӶԻ��������ж�Cԭ���ӻ����ͣ�
��3���÷�Ӧ�ж��ѵĻ�ѧ���ǹ��ۼ����γɵĻ�ѧ������λ����
��4��������������������ܽ�Ƚ��ͣ�
��5��Cu��29��Ԫ�أ���ԭ�Ӻ�����29�����ӣ�ʧȥ4s��3d�ܼ��ϸ�һ����������ͭ���ӣ�ͭ������Χ������9�����ݴ���дͭ������Χ�����Ų�ʽ��
3d�ܼ���û��δ�ɶԵ��ӵ�������ˮ��������ɫ��
��6���ٸ��ݼ۵����Ų�ʽ֪��YԪ����ClԪ�أ��þ�����Cu������Ϊ4�������Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4������ͭ���������Ӹ���֮��Ϊ4��4=1��1���ݴ��жϻ�ѧʽ��
�ھ������=$\frac{\frac{M}{{N}_{A}}��4}{��}$�������ⳤ=$\root{3}{\frac{4M}{��{N}_{A}}}$��Cuԭ�Ӻ�Clԭ����̾���Ϊ�����峤��$\frac{1}{4}$�������峤=$\sqrt{3}��$$\root{3}{\frac{4M}{��{N}_{A}}}$��
��� �⣺��1���л���Ĺ��������Ƿ��ӣ��ɷ��ӹ��ɵľ����Ƿ��Ӿ��壬M���л�����ڷ��Ӿ��壬�ʴ�Ϊ�����Ӿ��壻
��2��Ԫ�صķǽ�����Խǿ����縺��Խ��ͬ����������ҵ縺�����ʵ縺��O��N��C��H���ɽṹ��ʽ��֪���۲���ӶԸ�����3��Cԭ�Ӳ���sp2�ӻ���Cԭ�Ӽ۲���ӶԸ�����4�IJ���sp3�ӻ����ʴ�Ϊ��O��N��C��H��sp2��sp3��
��3���÷�Ӧ�ж��ѵĻ�ѧ���ǹ��ۼ����γɵĻ�ѧ������λ������ѡ��be��
��4������M���γɷ����������ʹ�ܽ�ȼ�С���ʴ�Ϊ��M���γɷ����������ʹ�ܽ�ȼ�С��
��5��Cuԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s1����Cu2+����Χ�����Ų�ʽΪ3d9��Zn2+���ӵ�ԭ�Ӻ����Ų�ʽΪ1s22s22p63s23p63d10��d�������10�����ӣ���Zn2+ ��ɫ��Sc3+���ӵ�ԭ�Ӻ����Ų�ʽΪ1s22s22p63s23p6����d�������0���ӣ�����ȫ�գ���û����ɫ���ʴ�Ϊ��3d9��3d�����û��δ�ɶԵ��ӣ�3d����ϵ���Ϊȫ�ջ�ȫ������
��6���ٸ��ݼ۵����Ų�ʽ֪��YԪ����ClԪ�أ��þ�����Cu������Ϊ4�������Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4������ͭ���������Ӹ���֮��Ϊ4��4=1��1�������仯ѧʽΪCuCl��
�ʴ�Ϊ��CuCl��
�ھ������=$\frac{\frac{M}{{N}_{A}}��4}{��}$�������ⳤ=$\root{3}{\frac{4M}{��{N}_{A}}}$��Cuԭ�Ӻ�Clԭ����̾���Ϊ�����峤��$\frac{1}{4}$�������峤=$\sqrt{3}��$$\root{3}{\frac{4M}{��{N}_{A}}}$��Cuԭ�Ӻ�Clԭ����̾���Ϊ�����峤��$\frac{1}{4}$=$\frac{1}{4}��$$\sqrt{3}$��$\root{3}{\frac{4M}{��{N}_{A}}}$��1010pm=$\frac{\sqrt{3}}{4}\root{3}{\frac{4��99.5}{��{N}_{A}}}$��1010pm��
�ʴ�Ϊ��=$\frac{\sqrt{3}}{4}\root{3}{\frac{4��99.5}{��{N}_{A}}}$��1010��
���� ���⿼�����ʽṹ�����ʣ��漰�������㡢ԭ���ӻ���ԭ�Ӻ�������Ų����縺�ԡ������֪ʶ�㣬Ϊ��Ƶ���㣬��Ϥ��̯�����۲���ӶԻ������ۡ�Ԫ�������ɡ�����ԭ����֪ʶ�㼴�ɽ��ע�����������ͷ��Ӽ�����������ܽ��Ե�Ӱ�죬�ѵ��Ǿ������㣬��Ŀ�Ѷ��еȣ�

| A�� | ��һ����������ͨ��30 mLŨ��Ϊ10.0mol/L����������Ũ��Һ�У���������ʱ�����Һ���γ�NaCl��NaClO��NaClO3������ϵ��n��NaCl����n��NaClO����n��NaClO3������Ϊ11��2��1 | |
| B�� | ʵ���ҿ�������һ�ֽ���Al3+��K+��SO42-��NO3-��4�����ӣ���������Դ��ˮ��������ӣ�����Һ����4�����ӵ�Ũ�Ⱦ�Ϊ1mol/L | |
| C�� | HCl��FeCl3��Fe3O4��NaOH����ͨ���û���Ӧһ���õ�Ҳ��ͨ�����Ϸ�Ӧһ���õ� | |
| D�� | ʵ������������Ϊ�˼ӿ췴Ӧ���ʣ�����ϡH2SO4�еμ�����Cu��NO3��2��Һ |
| X | Y | |
| W | Q |
| A�� | Y������ϼ�Ϊ+6 | B�� | �����ӵİ뾶��W��Q��Y��X | ||
| C�� | �⻯����ȶ��ԣ�W��Y | D�� | ����������ˮ��������ԣ�W��Q |
| A�� | ������������ձ������ʯ���� | |
| B�� | ��ȥ�Ҵ��е����ᣬ����NaOH��Һ���Һ | |
| C�� | ����0.1mol•L-1NaCl��Һ��ʵ���У���������ƽ��ȡ5.85gNaCl | |
| D�� | ������ͭ��Һ����Ũ������ȴ�ᾧ�õ�CuSO4•5H2O���� |
| Ԫ�ش��� | L | M | Q | R | T |
| ԭ�Ӱ뾶/nm | 0.160 | 0.143 | 0.089 | 0.104 | 0.066 |
| ��Ҫ���ϼ� | +2 | +3 | +2 | +6��-2 | -2 |
| A�� | �⻯��ķе�ΪH2T��H2R | B�� | ������ϡ���ᷴӦ�Ŀ���ΪL��Q | ||
| C�� | M��T�γɵĻ����������ӻ����� | D�� | L2+��R2-�ĺ����������� |
| A�� | HCl��H2SO4�������� | B�� | K2CO3��K2O�������� | ||
| C�� | NaOH��Na2CO3�����ڼ� | D�� | H2O��O2������������ |
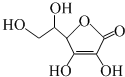
| A�� | ά����C�����Ĺ��������ǻ����Ȼ���̼̼˫�� | |
| B�� | ά����C�ܺ���ˮ�������ظ������Һ��Ӧ | |
| C�� | ά����C�ķ���ʽΪC6H6O6 | |
| D�� | ά����C�ܷ����ӳɷ�Ӧ��������Ӧ�����ܷ���ȡ����Ӧ |
| A�� | Y��Z��RԪ�ؼ����ӵİ뾶�������� | |
| B�� | ����Y��Z��R����Ԫ�صĻ��������ֻ��2�� | |
| C�� | Ԫ��W��R����������Ӧˮ��������Ժ���ǿ | |
| D�� | Y��Z�γɵ����ֻ������еĻ�ѧ�����ͺ��������Ӹ����Ⱦ���ͬ |