��Ŀ����
10������ͭ��������������Ҫ�Ļ���ԭ�ϣ���1��������ij�����ú����ķ�ͭΪԭ������������CuSO4•5H2O������������ʾ��ͼ��
������ʯ���ڲ�ͬ�¶��µ��ܽ�ȣ�g/100gˮ�����±���
| �¶ȣ��棩 | 20 | 40 | 60 | 80 | 100 |
| ʯ�� | 0.32 | 0.26 | 0.15 | 0.11 | 0.07 |
| ���� | 32 | 44.6 | 61.8 | 83.8 | 114 |
�ٺ��ɫ��������Ҫ�ɷ���Fe��OH��3��
��д��������������������ͭ�Ļ�ѧ����ʽ3Cu+8HNO3=3Cu��NO3��2+2NO��+4H2O��
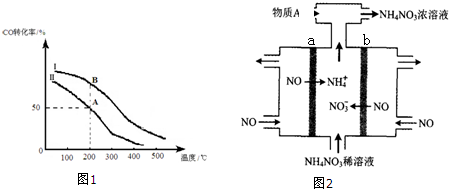
�۲���I���¶�Ӧ�ÿ�����100�����ң�
�ܴ���Һ�з��������ͭ����IJ���IIӦΪ������Һ��ȴ�ᾧ�����ˡ�ϴ�ӡ����
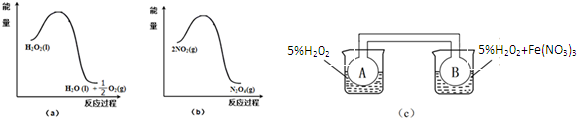
��2��ij��ȤС����ʵ��������ͼ��a���ͣ�b���е���Ϣ����ͼ��c��װ�ã���ͨ��A��Bƿ���ѳ���NO2���壩����Fe��NO3��3��H2O2�ֽ�����Ӱ���ʵ�飮5min��ɹ۲쵽Bƿ��������ɫ��Aƿ�е������dz��������ԭ����Fe��NO3��3��H2O2�ֽⷴӦ��������ã���ͼa֪H2O2�ķֽⷴӦΪ���ȷ�Ӧ����ͼbҲ֪2NO2?N2O4��ӦΪ���ȷ�Ӧ��Bƿ��H2O2��Fe��NO3��3�����������·ֽ�죬��ͬʱ���ڷ��ȶ࣬���Bƿ�����¶ȸߣ�2NO2?N2O4ƽ�������ƶ���NO2Ũ�ȴ���ɫ�
���� ��1�������ķ�ͭΪԭ�ϼ���ϡ�����ϡ����Ļ����Һ���ܽ��õ�����Һ���ڽ���Һ����Ҫ����Cu2+��Fe3+��H+��SO42-������ʯ�ҽ�������ҺPH���������ӣ����˵õ����ɫ����Ϊ������������������ʯ����������ܽ�ȣ�����100��C����Һ������ʯ�࣬��Һ����ҪΪ����ͭ��ͨ����������Ũ������ȴ�ᾧ������ϴ�ӣ�����õ�����ͭ���壻
���ɹ�������ͼת����ϵ��֪�����ɫ��������Ҫ�ɷ�Ϊ����������
���ɹ�������ͼת����ϵ��֪����������Ĵ��ڣ������������ȫ�����������ã���Ϊϡ��Һ����������ͭ��NO��ˮ��
���ɱ����ܽ�ȹ�ϵ��֪�������ܽ�����¶���������ʯ����ܽ�����¶����߽��ͣ�����Ӧ�����ڽϸߵ��¶ȣ����ʷ������ȫ���Ʊ��ĵ����ϴ���
�ܴ���Һ�з��������ͭ����ӦΪ������Һ��ȴ�ᾧ�����ˡ�ϴ�ӡ����
��2����ͼa��֪��1mol������������������1molˮ��0.5mol�������������ʹ�������ֽ��Ƿ��ȷ�Ӧ����ͼb��֪��2mol������������������1mol�������������������ʶ�������ת��Ϊ�����������ķ�ӦΪ���ȷ�Ӧ������ͼc�У��Ҳ��ձ����¶ȸ�����࣬�����¶�ʹ2NO2������ɫ��?N2O4����ɫ����H��0�����淴Ӧ�����ƶ���
��� �⣺��1�����ɹ�������ͼת����ϵ��֪������Һ�м���ʯ�ҽ�����pHֵ��������ת��ΪFe��OH��3�������������ɫ��������Ҫ�ɷ�ΪFe��OH��3��
�ʴ�Ϊ��Fe��OH��3��
����������Ĵ��ڣ��������������ȫ�����������ã���Ϊϡ��Һ����������ͭ��NO��ˮ����Ӧ����ʽΪ3Cu+2HNO3+3H2SO4=3CuSO4+2NO��+4H2O��
�ʴ�Ϊ��3Cu+2HNO3+3H2SO4=3CuSO4+2NO��+4H2O��
���ɱ����ܽ�ȹ�ϵ��֪�������ܽ�����¶���������ʯ����ܽ�����¶����߽��ͣ�����Ӧ�����ڽϸߵ��¶ȣ��¶�Ӧ�ÿ�����100�棬�Ʊ��ĵ�����Խϴ���
�ʴ�Ϊ��100�棻
�ܴ���Һ�з��������ͭ����ӦΪ������Һ��ȴ�ᾧ�����ˡ�ϴ�ӡ�����ʴ�Ϊ����ȴ�ᾧ�����ˣ�
��2����ͼa��֪��1mol������������������1molˮ��0.5mol�������������ʹ�������ֽ��Ƿ��ȷ�Ӧ����ͼb��֪��2mol������������������1mol�������������������ʶ�������ת��Ϊ�����������ķ�ӦΪ���ȷ�Ӧ��Fe��NO3��3��H2O2�ֽⷴӦ��������ã���ͼa֪H2O2�ķֽⷴӦΪ���ȷ�Ӧ����ͼbҲ֪2NO2?N2O4 ��ӦΪ���ȷ�Ӧ��Bƿ��H2O2��Fe��NO3��3�����������·ֽ�죬��ͬʱ���ڷ��ȶ࣬���Bƿ�����¶ȸߣ�2NO2?N2O4 ƽ�������ƶ���NO2Ũ�ȴ���ɫ���
�ʴ�Ϊ���Fe��NO3��3��H2O2�ֽⷴӦ��������ã���ͼa֪H2O2�ķֽⷴӦΪ���ȷ�Ӧ����ͼbҲ֪2NO2?N2O4 ��ӦΪ���ȷ�Ӧ��Bƿ��H2O2��Fe��NO3��3�����������·ֽ�죬��ͬʱ���ڷ��ȶ࣬���Bƿ�����¶ȸߣ�2NO2?N2O4 ƽ�������ƶ���NO2Ũ�ȴ���ɫ���
���� ���⿼��ѧ���Թ������̵����⡢�Ķ���Ŀ��ȡ��Ϣ���������ʷ����ᴿ�Ȼ�����������ͼ��������ѧƽ��Ӱ�����ط�������ƽ���ƶ�ԭ����Ӧ�õȣ��Ѷ��еȣ�Ҫ��ѧ��Ҫ����ʵ��ʵ�����֪ʶ�����Ӧ����Ϣ������֪ʶ��������������

 ��У����ϵ�д�
��У����ϵ�д�
| A�� | ������������4��̼ԭ�Ӵ���ͬһֱ���� | |
| B�� | ���������ϵ�һ�ȴ�����4�� | |
| C�� | ������������10��̼ԭ�Ӵ���ͬһƽ���� | |
| D�� | �����DZ���ͬϵ�� |
| A�� | $\frac{{K}_{W}}{c��{H}^{+}��}$=0.1mol•L-1����Һ��Na+��SiO32-��I-��CO32- | |
| B�� | 2%�İ�ˮ��Ag+��K+��F-��NO3- | |
| C�� | pH=1����Һ��NH4+��Na+��SO42-��C17H35COO- | |
| D�� | 0.1mol•L-1��NaHSO4��Һ��K+��Fe2+��Cl-��CrO42- |
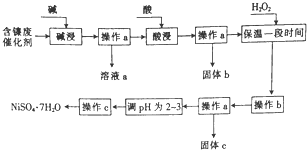 ij��ѧС��ͨ���������ϣ��������ͼ��ʾ�ķ����Ժ����ϴ���Ϊԭ�����Ʊ�NiSO4•7H2O����֪ij�������ĺ����ϴ�����Ҫ����Ni��������Al��31%����Fe��1.3%���ĵ��ʼ�����������������ʣ�3.3%����
ij��ѧС��ͨ���������ϣ��������ͼ��ʾ�ķ����Ժ����ϴ���Ϊԭ�����Ʊ�NiSO4•7H2O����֪ij�������ĺ����ϴ�����Ҫ����Ni��������Al��31%����Fe��1.3%���ĵ��ʼ�����������������ʣ�3.3%��������������������������ʽ����ʱ��pH���£�
| ������ | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Al��OH��3 | 3.8 | 5.2 |
| Fe��OH��3 | 2.7 | 3.2 |
| FE��OH��2 | 7.6 | 9.7 |
| Ni��OH��2 | 7.1 | 9.2 |
��2��������������з�����Ӧ�����ӷ���ʽ��2Al+2OH-+2H2O�T2AlO2-+3H2����Al2O3+2OH-�T2AlO2-+H2O��
��3������bΪ������Һ��pH������ΪpH�ĵ��ط�Χ��3.2-7.1��
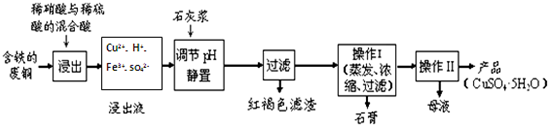
��4����Һa�м���������Ƶ�AlCl3��Һ��AlCl3��Һ�������Ʊ��ۺ��Ȼ������ۺ��Ȼ�����һ�����;�ˮ������������Ҫ��[AlO4Al12��OH��2��H2O��12]2+����Alb��ʾ������ʽ���ڣ�
��д������Һa�����ᷴӦ�Ʊ�AlCl3�����ӷ���ʽ��AlO2-+4H+=Al3++2H2O��
��һ�������£���1.0mol•L-1��AlCl3��Һ�м���0.6mol•L-1��NaOH��Һ�����Ƶ�Alb����ԼΪ86%�ľۺ��Ȼ�����Һ��д������[AlO4Al12��OH��24��H2O��22]2+�����ӷ���ʽ��13Al3++32OH-+8H2O=[AlO4Al12��OH��24��H2O��12]7+��
| A�� | �÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��K=$\frac{{c}^{2}��C��•{c}^{2}��D��}{c��A��•c��B��}$ | |
| B�� | ����ƽ����ϵ�м�������������C���������淴Ӧ���ʾ����� | |
| C�� | �������ϵ��ѹǿ��A��ת�������� | |
| D�� | ��������B��ƽ��ת������40% |
��1����֪25�桢101kPaʱ��
2SO2��g��+O2��g��?2SO3��g����H1=-197kJ•mol-1
H2O��g��=H2O��l����H2=-44kJ•mol-1
2SO2��g��+O2��g��+2H2O��g��=2H2SO4��l����H3=-545kJ•mol-1
��SO3��g����H2O��l����Ӧ���Ȼ�ѧ����ʽ��SO3��g��+H2O��l���T2H2S O4��l����H=-130 kJ/mol��
��2������Ӧ2H2��g��+O2��g���T2H2O��g ������H=-241.8kJ•mol-1�������±����ݣ���x=738.2 kJ•mol-1��
| ��ѧ�� | H-H | O�TO | O-H |
| �Ͽ�1mol��ѧ�����������/kJ | 436 | x | 463 |
�ٸ��¶��£��������ݻ���Ϊ1L���ܱ������У��ֱ����÷�Ӧ��
| ���� | �� | �� |
| ��Ӧ��Ͷ���� | 1mol CO ��g����2mol H2��g�� | 1mol CH3OH��g�� |
| ƽ��ʱc��CH3OH�� | c1 | c2 |
| ƽ��ʱ�����仯 | �ų�54kJ | ����a kJ |
�����ܱ������ݻ������ͬ�������ߣ�ͼ1���ֱ��ʾͶ�ϱȲ�ͬʱ�ķ�Ӧ���̣� ����Ӧ��n��CO����ʼ=10mol��Ͷ�ϱ�Ϊ0.5����A���ƽ�ⳣ��KA=0.01��B���ƽ�ⳣ��KB=KA���������������=����
��Ϊ���COת���ʿɲ�ȡ�Ĵ�ʩ�Ǽ�СͶ�ϱȣ������¶ȣ�����ѹǿ�������CH3OH�ȣ����ٴ����������
��4�����NO�Ʊ�NH4NO3���乤��ԭ����ͼ2��ʾ����a�缫����Ϊ������b�缫��ӦʽΪNO+2H2O-3e-=NO3-+4H+��