��Ŀ����
15����ҵ�ϳ��ӵ�⾫��ͭ������������ȡ���������仯�����ڵ����¿ɱ�����ΪSeO2���������K������ˮ�γ�H2SeO3����˹�ҵ���á���������һ�������ȡ�������������£�
��1�������Һ��ͨ��SO2����ԭ���ô�����д����ѧ��Ӧ����ʽH2SeO3+2SO2+H2O=2H2SO4+Se���ɴ�֪�����е�H2SO4��ѭ��ʹ�ã�
��2����Se����ʱ��Se�������������б�������SeO2�������������γ�ѩ��ɫ��SeO2��ĩ���ڴ˹���������������Ϊ�������������壮
��3���������������������·����ⶨ��
��Se+2H2SO4��Ũ��=2SO2��+SeO2+2H2O
��SeO2+4KI+4HNO3=Se+2I2+4KNO3+2H2O
��l2+2Na2S2O3=Na2S4O6+2Nal����֪H2S2O3Ϊ���ᣩ
ͨ����Na2S2O3��Һ�ζ���Ӧ�����ɵ�l2���������ĺ�����Na2S2O3��ҺӦ�ü�ʽ�����ʽ����ʽ�����ζ�����ȡ��ʵ����Ҫ���������Һ��Ϊָʾ����ʵ�鵽���յ�ʱ���������һ�Σ���ɫǡ����ʧ�Ұ�����ڲ���ɫ��
��4����֪0.1200g������Ʒ���ζ�����0.2000mol•L-1����Һ30.00ml�����������������������98.75%��
���� �������к���Ag��Au��Cu��Se��CuSe��Ag2Se���ڿ����б��յõ��ı������к�SeO2����������������Һ�����˵õ�������ҪΪAg��Au��CuO����Һ����Һ�еõ�NaSeO3�����������ữ�õ�H2SeO3��ͨ��SO2��ԭ�ô�����
��1�������Һ��ͨ��SO2����ԭ���ô��������ö�������Ļ�ԭ�Ի�ԭ������ΪSe��������������Ϊ���ᣬ�����м����������������ԭ��Ӧ�л�������ɣ�����ѭ��ʹ�ã�
��2����Se����ʱ��Se�������������б�������SeO2�������������γ�ѩ��ɫ��SeO2��ĩ��������Ҫ�������������������Ҫ���ã�
��3��Na2S2O3��Һ�Լ���ѡ���ʽ�ζ��ܣ�ͨ����Na2S2O3��Һ�ζ���Ӧ�����ɵ�l2���������ĺ���������ѡ��ָʾ��Ϊ������Һ�����뵽���һ����ɫǡ����ʧ�Ұ���Ӳ���ɫ��
��4�����ݷ�Ӧ�ķ���ʽ��֪��SeO2��2I2��4Na2S2O3������n=cV�������ĵ�n��Na2S2O3�������ݹ�ϵʽ������Ʒ��n��SeO2�����ٸ���m=nM����SeO2������������������Ʒ��Se������������
��� �⣺��1�������Һ��ͨ��SO2����ԭ���ô��������ö�������Ļ�ԭ�Ի�ԭ������ΪSe��������������Ϊ���ᣬ��Ӧ�Ļ�ѧ����ʽΪ��H2SeO3+2SO2+H2O=2H2SO4+Se����Ӧ���������ᣬ�����м����������������ԭ��Ӧ�л�������ɣ�����ѭ��ʹ�ã�
�ʴ�Ϊ��H2SeO3+2SO2+H2O=2H2SO4+Se��H2SO4��
��2�����ݴ�Se����ʱ��Se�������������б�������SeO2�������������γ�ѩ��ɫ��SeO2��ĩ������֪��������Ҫ�������������������Ҫ���ã�
�ʴ�Ϊ���������������壻
��3��Na2S2O3����Һ�Լ���Ӧ�ü�ʽ�ζ���ʢ�ţ�������Һ����ƿʢ�ţ�ͨ����Na2S2O3��Һ�ζ���Ӧ�����ɵ�l2���������ĺ���������ѡ��ָʾ��Ϊ������Һ�����뵽���һ����ɫǡ����ʧ�Ұ���Ӳ���ɫ��
�ʴ�Ϊ����ʽ��������Һ�����һ�Σ���ɫǡ����ʧ�Ұ�����ڲ���ɫ��
��4�����ݷ�Ӧ�ķ���ʽ��֪Se��SeO2��2I2��4Na2S2O3�����ĵ�n��Na2S2O3��=0.2000 mol/L��0.030L=0.006mol�����ݹ�ϵʽ������Ʒ��n��Se��=0.006mol��$\frac{1}{4}$=0.0015mol����Se������Ϊ0.0015mol��79g/mol=0.1185g��������Ʒ��Se����������Ϊ$\frac{0.1185g}{0.1200g}$��100%=98.75%��
�ʴ�Ϊ��98.75%��
���� �����Թ�������ͼΪ֪ʶ������������������ԭ��Ӧ�����Ӽ��顢����ʵ����������ʴ��ȵIJⶨ�������ȣ���Ŀ�漰��֪ʶ��϶࣬����ѧ����֪ʶ���ۺ�Ӧ����������Ŀ�Ѷ��еȣ�

 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д�| A�� | ���ǵ�������������Ӧˮ����ļ������μ���������������ǿ | |
| B�� | ���ǵ�ԭ�Ӱ뾶�������� | |
| C�� | ���ǵĵ����ڳ��£���ѹ�µ��ܶ����μ�С | |
| D�� | ���ǵ�����������ˮ���ﶼ��ǿ����� |
| A�� | c��H+�����¶ȵ����߶����� | |
| B�� | 35��ʱ��c��H+����c��OH-�� | |
| C�� | ��ҺpH��pH��35�棩��pH��25�棩 | |
| D�� | 35��ʱ�ѵ����ˮ��Ũ��ԼΪ1.45��10-7 mol/L |
��1��������ij�����ú����ķ�ͭΪԭ������������CuSO4•5H2O������������ʾ��ͼ��
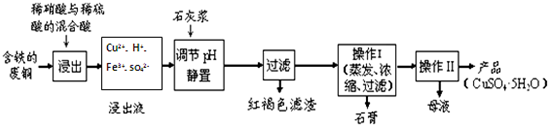
������ʯ���ڲ�ͬ�¶��µ��ܽ�ȣ�g/100gˮ�����±���
| �¶ȣ��棩 | 20 | 40 | 60 | 80 | 100 |
| ʯ�� | 0.32 | 0.26 | 0.15 | 0.11 | 0.07 |
| ���� | 32 | 44.6 | 61.8 | 83.8 | 114 |
�ٺ��ɫ��������Ҫ�ɷ���Fe��OH��3��
��д��������������������ͭ�Ļ�ѧ����ʽ3Cu+8HNO3=3Cu��NO3��2+2NO��+4H2O��
�۲���I���¶�Ӧ�ÿ�����100�����ң�
�ܴ���Һ�з��������ͭ����IJ���IIӦΪ������Һ��ȴ�ᾧ�����ˡ�ϴ�ӡ����
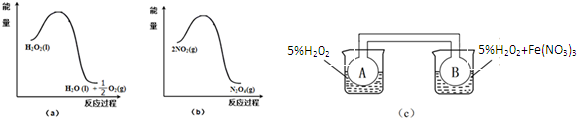
��2��ij��ȤС����ʵ��������ͼ��a���ͣ�b���е���Ϣ����ͼ��c��װ�ã���ͨ��A��Bƿ���ѳ���NO2���壩����Fe��NO3��3��H2O2�ֽ�����Ӱ���ʵ�飮5min��ɹ۲쵽Bƿ��������ɫ��Aƿ�е������dz��������ԭ����Fe��NO3��3��H2O2�ֽⷴӦ��������ã���ͼa֪H2O2�ķֽⷴӦΪ���ȷ�Ӧ����ͼbҲ֪2NO2?N2O4��ӦΪ���ȷ�Ӧ��Bƿ��H2O2��Fe��NO3��3�����������·ֽ�죬��ͬʱ���ڷ��ȶ࣬���Bƿ�����¶ȸߣ�2NO2?N2O4ƽ�������ƶ���NO2Ũ�ȴ���ɫ�
������ͭ˿����Ũ���ᣬ���ȣ�
������������ɫ����������ʱ�����ͭ˿��ֹͣ���ȣ�
����ȴ�ӷ�Ӧ��Ļ�����з������ɫ������ϴ�������ﱸ�ã�
��1��������У����ӷ�Ӧ��Ļ�����з������ɫ�������IJ����ǽ���Ӧ��Ļ���ﵹ��װ����ˮ���ձ��У���ȴ����ˣ�
��2����ͬѧ�����ɫ������CuO������������£�
�������ף�������Cu2+�ķ����ǣ�����Һ�еμ�K4[Fe��CN��6]��Һ�����������ɫ������֤����Cu2+��
�ٽ�CuO����ϡ�����У�һ��ʱ���δ�����������ٵμ�K4[Fe��CN��6]��Һ���������ɫ������
�ڽ���ɫ��������ϡ�����У�һ��ʱ��μ�K4[Fe��CN��6]��Һ��δ�����ɫ������
�ɸü���������ý����Ǻ�ɫ�����в�����CuO��
��3���ٴμ��裬��ɫ������ͭ�����ʵ�����£�
| ʵ��װ�� | ���� |
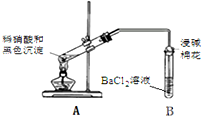 | 1��A�Թ��к�ɫ�������ܽ� 2��A�Թ����Ϸ����ֺ���ɫ���� 3��B�Թ��г��ְ�ɫ���� |
����ȷ�Ϻ�ɫ�����к���SԪ�ص�������B�Թ��г��ְ�ɫ��������Ӧ�����ӷ���ʽ��NO2+SO2+Ba2++H2O�TBaSO4��+NO��+2H+��
��Ϊȷ�Ϻ�ɫ�����ǡ�ͭ�������������е�ʵ����ȡ��ȴ��Aװ���Թ��е���Һ���μ�K4[Fe��CN��6]��Һ�����������ɫ������֤����Cu2+��˵����ɫ������ͭ�����
��4������ʵ��˵������ɫ�����д���ͭ�������һ��ʵ���֤����ɫ������CuS��Cu2S�Ļ�������ɫ��������Ũ�����м���һ��ʱ������ܽ⣬����CuS�ܽ�Ļ�ѧ����ʽ��CuS+4H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+4SO2��+4H2O��
| A�� | C�������������� | B�� | ƽ�������ƶ� | C�� | B��ת�������� | D�� | x+y��z |
| A�� | 1mol/L Na2CO3��Һ�е�Na+��ĿΪ2NA | |
| B�� | 1 mol Na������O2��Ӧ����Na2O��Na2O2��ʧȥNA���� | |
| C�� | ��״���£�11.2LSO3����������Ϊ0.5NA | |
| D�� | ��5.6 g���ֱ������������ᡢ������Ӧ������ת��������Ϊ0.3NA |
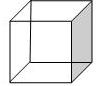 �������顱��һ�r�ºϳɵ����������Ϊ��������ṹ����̼�ܽṹ��ͼ��ʾ
�������顱��һ�r�ºϳɵ����������Ϊ��������ṹ����̼�ܽṹ��ͼ��ʾ ��
��