��Ŀ����
����Ŀ���о�CO2�����öԴٽ���̼���Ĺ���������Ҫ�����塣
��1����ȼú�����е�CO2ת��Ϊ�����ѵķ�Ӧԭ��Ϊ��2CO2(g)+ 6H2(g) ![]() CH3OCH3(g) + 3H2O(l)���÷�Ӧ��ѧƽ�ⳣ������ʽK = ________________________��
CH3OCH3(g) + 3H2O(l)���÷�Ӧ��ѧƽ�ⳣ������ʽK = ________________________��
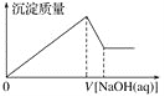
��2����֪��ijѹǿ�£��÷�Ӧ�ڲ�ͬ�¶ȡ���ͬͶ�ϱ�ʱ����ƽ��ʱCO2��ת������ͼ
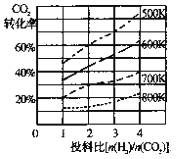
�ٸ÷�Ӧ����H ________ 0������>"����<������
�����¶Ȳ��䣬��С��ӦͶ�ϱ�[n(H2)/n(CO2)]��Kֵ��________������������������С����������������
��3��ij�¶��£������һ�����ܱ�������ͨ��CO2(g)��H2(g)����������Ӧ���������������ٷ����仯ʱ����˵����Ӧ�ﵽƽ��״̬����__________��
A.��������ɫ B.�����е�ѹǿ
C.������ܶ� D.CH3OCH3��H2O�����ʵ���֮��
��4��ij�¶��£�������ɱ���ܱ������У��ı���ʼʱ��������ʵ������ڲ�ͬ��ѹǿ�£�ƽ��ʱCH3OCH3(g)�����ʵ������±���ʾ��
P1 | P2 | P3 | |
I��2.0 mol CO2 6.0 mol H2 | 0.10 mol | 0.04 mol | 0.02 mol |
II��1.0 mol CO2 3.0 mol H2 | X1 | Y1 | Z1 |
III��1.0 mol CH3OCH3 3.0 mol H2O | X2 | Y2 | Z2 |
��P1 ________ P3������>����<������=������
��P2�£�III��CH3OCH3��ƽ��ת����Ϊ__________��
���𰸡�![]() �� ���� B,C �� 96%
�� ���� B,C �� 96%
��������
��1������ƽ�ⳣ�������������Ũ����֮�����Է�Ӧ���Ũ����֮��д����ʽ��
��2���ٸ����¶ȶ�ƽ���Ӱ�������H�ķ��ţ�
��ƽ�ⳣ��Kֻ���¶��йأ�
��3�������淴Ӧ�ﵽƽ��״̬ʱ�����淴Ӧ������ȣ���Ӧ�����и����ʵ����ʵ��������ʵ���Ũ�ȼ��ٷֺ��������䣬�Լ��ɴ������һЩ���������䣬�ݴ˷������
��4����ӦΪ���������С�ķ�Ӧ������ѹǿƽ��������У�
��1.0molCH3OCH3��3.0molH2O����ʼ��2.0molCO2��6.0molH2 ��ȴﵽ��ͬ��ƽ��״̬���ݴ���ʽ���㣮
(1)ƽ�ⳣ�������������Ũ����֮�����Է�Ӧ���Ũ����֮��,����ƽ�ⳣ��K=![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(2)����Ϊ�¶�Խ�ߣ�CO2ת����ԽС����ƽ�������ƶ������Ը÷�Ӧ������Ϊ���ȷ�Ӧ������H��0���ʴ�Ϊ������
��Kֻ���¶�Ӱ�죬���¶Ȳ��䣬��СͶ�ϱȣ���K���䣬�ʴ�Ϊ�����䣻
(3)A.��Ӧ��ϵ��û����ɫ���壬�ʻ��������ɫʼ�ղ��䣬��A��ѡ��
B. ��Ӧǰ�������ϵ���Ͳ���ȣ���������ѹǿ���ٸı䣬��ﵽ��ƽ�⣬��Bѡ��
C. �÷�Ӧ��һ����Ӧǰ����������仯�Ŀ��淴Ӧ��������������䣬����Ӧǰ�����������仯��������ܶȲ������仯����ﵽ��ƽ�⣬��Cѡ��
D. �κ�ʱ��CH3OCH3��H2O�����ʵ���֮�Ȳ��䣬����˵����Ӧ�ﵽƽ�⣬��D��ѡ��
�ʴ�Ϊ��BC��
(4)��2CO2(g)+ 6H2(g) ![]() CH3OCH3(g) + 3H2O(l),��ӦΪ���������С�ķ�Ӧ,����ѹǿƽ��������У�CH3OCH3�����ʵ�������ͼ����ƽ��ʱCH3OCH3(g)�����ʵ�����֪P1>P2��
CH3OCH3(g) + 3H2O(l),��ӦΪ���������С�ķ�Ӧ,����ѹǿƽ��������У�CH3OCH3�����ʵ�������ͼ����ƽ��ʱCH3OCH3(g)�����ʵ�����֪P1>P2��
�ʴ�Ϊ��>��
��I��ʼ����2.0molCO2��6.0molH2��P2��ƽ�����м��㣬

ƽ��ʱCO2��ת����Ϊ4%����ʼ��1.0molCH3OCH3��3.0molH2O����ʼ��2.0molCO2��6.0molH2 ��ȴﵽ��ͬ��ƽ��״̬������Ͷ�ϴﵽƽ��ʱ��Ӧ���ת����֮��Ϊ1������P2�¢���CH3OCH3��ƽ��ת����=1-4%=96%��
�ʴ�Ϊ��96%.

 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�����Ŀ����1����д���б���
���� | ������ | ����/g | ���ʵ���/ mol | Ħ������/(g |
O2 | __ | 8.0 | __ | __ |
H2SO4 | 3.01��1023 | __ | __ | __ |
H2O | __ | __ | 0.5 | __ |
��2��147gH2SO4�����ʵ�����____��0.5molH2SO4��������____g�����к���____mol H��2 mol H2SO4�к���H2SO4������Ϊ_____��������ԭ����Ϊ____����
��3��12.4gNa2R��Na��0.4mol����Na2R��Ħ������Ϊ____��R�����ԭ������Ϊ____����R������Ϊ1.6 g��Na2R�������ʵ���Ϊ____��