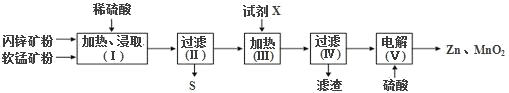
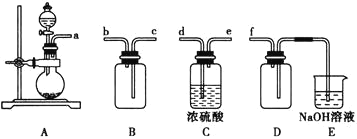
��Ŀ����
����Ŀ��A��B��C��D��E��F�Ƕ���������Ԫ�أ���ԭ���������������ڶ�������AԪ��ԭ�Ӱ뾶��С��DԪ��ԭ�Ӱ뾶���B�ļ��⻯���ˮ��Һ�ʼ��ԣ�C��Eͬ���壬�γɵĻ�����Ϊ![]() ��
��![]() ���ش��������⣺
���ش��������⣺
![]() ��Ԫ�����ڱ��е�λ��Ϊ_______��
��Ԫ�����ڱ��е�λ��Ϊ_______��
![]() �Ƚ�B��C���⻯������ȶ��ԣ�_____>____��
�Ƚ�B��C���⻯������ȶ��ԣ�_____>____��![]() �ѧʽ
�ѧʽ![]()
![]() ��C��Ԫ����ɵĻ�����
��C��Ԫ����ɵĻ�����![]() ��ˮ��Ӧ�Ļ�ѧ����ʽΪ__________
��ˮ��Ӧ�Ļ�ѧ����ʽΪ__________
![]() д��ʵ������ȡBA3�Ļ�ѧ����ʽ__________
д��ʵ������ȡBA3�Ļ�ѧ����ʽ__________
(5)ʵ���Ҽ���BA3�ķ���_________
(6)D��F������������ˮ�������Խ�ǿ����_________(�û�ѧʽ��ʾ)
(7)�õ���ʽ��ʾ![]() _________
_________![]() _____________
_____________
(8)����˵����ȷ����__________
A��Ԫ��F�γɵĵ��ʱ�Ԫ��E�γɵĵ��ʵ��۵��
B��F��E��Ԫ�صļ��⻯�����ȷֽ⣬ǰ�ߵķֽ��¶ȸ�
C��![]() ͨ�뵽
ͨ�뵽![]() ����Һ�г��ֻ���
����Һ�г��ֻ���
D��F�⻯������Ա�E�⻯�������ǿ
���𰸡���3���ڵ���A�� ![]()
![]()
![]() 2NH4Cl+Ca(OH)2
2NH4Cl+Ca(OH)2![]() CaCl2+2NH3��+2H2O ����ʪ�ĺ�ɫʯ����ֽ���뼯��ƿ������ֽ������˵��Ϊ���� HClO4
CaCl2+2NH3��+2H2O ����ʪ�ĺ�ɫʯ����ֽ���뼯��ƿ������ֽ������˵��Ϊ���� HClO4 ![]()
![]()
![]()
��������
�ڶ�������AԪ��ԭ�Ӱ뾶��С��DԪ��ԭ�Ӱ뾶���A��HԪ�ء�D��NaԪ�أ�B�ļ��⻯���ˮ��Һ�ʼ��ԣ�B��NԪ�أ�C��Eͬ���壬�γɵĻ�����Ϊ![]() ��
��![]() ����C��OԪ�ء�E��SԪ�أ�A��B��C��D��E��F�Ƕ���������Ԫ�أ���ԭ����������������F��ClԪ�ء�
����C��OԪ�ء�E��SԪ�أ�A��B��C��D��E��F�Ƕ���������Ԫ�أ���ԭ����������������F��ClԪ�ء�
(1)![]() ��SԪ�أ���Ԫ�����ڱ��е�λ��Ϊ��3���ڵ���A�塣
��SԪ�أ���Ԫ�����ڱ��е�λ��Ϊ��3���ڵ���A�塣
(2)ͬ����Ԫ�ش����ҷǽ�������ǿ���ǽ�����N��O�����⻯������ȶ���H2O��NH3��
(3)������![]() ��ˮ��Ӧ�����������ƺ���������Ӧ�Ļ�ѧ����ʽΪ
��ˮ��Ӧ�����������ƺ���������Ӧ�Ļ�ѧ����ʽΪ![]() ��
��
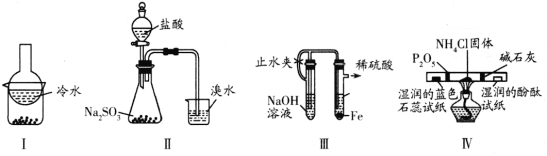
(4)ʵ�������Ȼ�李��������ƻ�ϼ�����ȡ��������Ӧ�Ļ�ѧ����ʽ��2NH4Cl+Ca(OH)2![]() CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O��
(5)������ˮ��Һ�Լ��ԣ�������ʹʪ��ĺ�ɫʯ����ֽ����������ʪ�ĺ�ɫʯ����ֽ���뼯��ƿ������ֽ������˵��Ϊ������
(6)Na������������ˮ������NaOH��Cl������������ˮ������HClO4�����Խ�ǿ����HClO4��
(7) ![]() �����ӻ��������ʽ��
�����ӻ��������ʽ��![]() ��
��![]() �ǹ��ۻ��������ʽ��
�ǹ��ۻ��������ʽ��![]() ��
��
(8) A��������۵����������۵㣬����SΪ���壬���۵�������ߣ���A����
B��ͬ����Ԫ�ش����ҷǽ�������ǿ���ǽ�����S��Cl���ȶ���HCl��H2S�����Լ��⻯�����ȷֽ⣬HCl�ķֽ��¶ȸߣ���B��ȷ��
C������ͨ�뵽![]() ��Һ�������Ȼ��ƺ����������C��ȷ��
��Һ�������Ȼ��ƺ����������C��ȷ��
D������Ϊǿ�ᣬ��������Ϊ���ᣬ����HClˮ��Һ�����Ա�H2Sˮ��Һ������ǿ����D����
ѡBC��

 �ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д�
�ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д�����Ŀ���о�CO2�����öԴٽ���̼���Ĺ���������Ҫ�����塣
��1����ȼú�����е�CO2ת��Ϊ�����ѵķ�Ӧԭ��Ϊ��2CO2(g)+ 6H2(g) ![]() CH3OCH3(g) + 3H2O(l)���÷�Ӧ��ѧƽ�ⳣ������ʽK = ________________________��
CH3OCH3(g) + 3H2O(l)���÷�Ӧ��ѧƽ�ⳣ������ʽK = ________________________��
��2����֪��ijѹǿ�£��÷�Ӧ�ڲ�ͬ�¶ȡ���ͬͶ�ϱ�ʱ����ƽ��ʱCO2��ת������ͼ
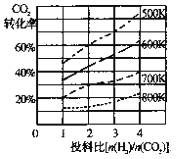
�ٸ÷�Ӧ����H ________ 0������>"����<������
�����¶Ȳ��䣬��С��ӦͶ�ϱ�[n(H2)/n(CO2)]��Kֵ��________������������������С����������������
��3��ij�¶��£������һ�����ܱ�������ͨ��CO2(g)��H2(g)����������Ӧ���������������ٷ����仯ʱ����˵����Ӧ�ﵽƽ��״̬����__________��
A.��������ɫ B.�����е�ѹǿ
C.������ܶ� D.CH3OCH3��H2O�����ʵ���֮��
��4��ij�¶��£�������ɱ���ܱ������У��ı���ʼʱ��������ʵ������ڲ�ͬ��ѹǿ�£�ƽ��ʱCH3OCH3(g)�����ʵ������±���ʾ��
P1 | P2 | P3 | |
I��2.0 mol CO2 6.0 mol H2 | 0.10 mol | 0.04 mol | 0.02 mol |
II��1.0 mol CO2 3.0 mol H2 | X1 | Y1 | Z1 |
III��1.0 mol CH3OCH3 3.0 mol H2O | X2 | Y2 | Z2 |
��P1 ________ P3������>����<������=������
��P2�£�III��CH3OCH3��ƽ��ת����Ϊ__________��