��Ŀ����
������ʵ��д�����з�Ӧ���Ȼ�ѧ��Ӧ����ʽ��
��1����25�桢101kPa�£�1g�״���ȫȼ������CO2��Һ̬ˮʱ����22��68kJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ ��
��2����������N2��O2��ȫ��Ӧ��ÿ����23gNO2��Ҫ����16��95kJ���������ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��3����NA��ʾ�����ӵ���������C2H2����̬����ȫȼ������CO2��Һ̬ˮ�ķ�Ӧ�У�ÿ��5NA������ת��ʱ���ų�650kJ�����������ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ _________________________________________________��
��4����֪��1molH��H����1molN��H����1molN��N���ֱ���Ҫ��������436kJ��391kJ��946kJ����N2��H2��Ӧ����NH3���Ȼ�ѧ����ʽΪ ��
��1��CH3OH��l��+1��5O2��g���TCO2��g��+2H2O��l����H=-725��8 kJ?mol-1��
��2��N2��g��+2O2��g��=2NO2��g����H=+67��8kJ?mol-1��
��3��C2H2��g��+O2��g����2CO2��g��+H2O��l����H=-1300kJ?mol-1��
��4��N2��g��+3H2��g�� 2NH3��g����H=-92kJ?mol-1��
2NH3��g����H=-92kJ?mol-1��
���������������1����25�桢101kPa�£�1g�״���CH3OH��ȼ������CO2��Һ̬ˮʱ����22��68kJ��1mol�״���ȫȼ�����ɶ�����̼��Һ̬ˮ����Ϊ22��68kJ��32=725��8KJ�����Լ״�ȼ���ȵ��Ȼ�ѧ����ʽΪCH3OH��l��+1��5O2��g���TCO2��g��+2H2O��l����H=-725��8 kJ?mol-1��
��2��������N2��O2��ȫ��Ӧ��ÿ����23��NO2��Ҫ����16��95kJ����������ÿ����92��NO2��Ҫ����67��8kJ���������Ȼ�ѧ����ʽΪN2��g��+2O2��g��=2NO2��g����H=+67��8kJ?mol-1��
��3����3����C2H2����̬����ȫȼ������CO2��Һ̬ˮ�ķ�Ӧ�У�ÿ��5NA������ת��ʱ���ų�650kJ��������������10NA������ת��ʱ���ų�1300kJ��������
���Ȼ�ѧ����ʽΪ��C2H2��g��+2��5O2��g����2CO2��g��+H2O��l����H=-1300kJ?mol-1��
��4���ڷ�ӦN2+3H2?2NH3�У�����3molH-H����1molN��N�������յ�����Ϊ3��436kJ+946kJ=2254kJ������2molNH3�����γ�6molN-H�����ų�������Ϊ6��391kJ=2346kJ�����յ������٣��ų��������࣬�÷�ӦΪ���ȷ�Ӧ���ų�������Ϊ2346kJ-2254kJ=92kJ��N2��H2��Ӧ����NH3���Ȼ�ѧ����ʽΪ��N2��g��+3H2��g�� 2NH3��g����H=-92kJ?mol-1��
2NH3��g����H=-92kJ?mol-1��
���㣺�Ȼ�ѧ����ʽ����д

���״���һ�����͵���������ȼ�ϣ���ҵ�Ͽ�ͨ��CO��H2�����Ʊ��״����÷�Ӧ���Ȼ�ѧ����ʽΪ��CO(g)+2H2(g) CH3OH(g) ��H
CH3OH(g) ��H
��֪ijЩ��ѧ���ļ����������±���
| ��ѧ�� | C��C | C��H | H��H | C��O | C��O | H��O |
| ����/kJ��mol��1 | 348 | 413 | 436 | 358 | 1072 | 463 |
��ش��������⣺
��1����֪CO�е�C��O֮��Ϊ�������ӣ��÷�Ӧ�ġ�H= ��
��2��ij��ѧ�о���ѧϰС��ģ�ҵ�ϳɼ״��ķ�Ӧ�����ݻ��̶�Ϊ2L���ܱ������ڳ���1molCO��2molH2��������ʴ�����������Ժ��Բ��ƣ�����250��C��ʼ��Ӧ������ѹ���Ƽ��������ѹǿ�ı仯���£�
| ��Ӧʱ��/min | 0 | 5 | 10 | 15 | 20 | 25 |
| ѹǿ/MPa | 12��6 | 10��8 | 9��5 | 8��7 | 8��4 | 8��4 |
��ӷ�Ӧ��ʼ��20minʱ����CO��ʾ��ƽ����Ӧ����= �����¶���ƽ�ⳣ��K= ���������¶���Kֵ �����������С�����䡱����
��3��������������˵��������Ӧ�Ѵ�ƽ����� ��
A��2v(H2)��=v(CH3OH)��
B�������������ƽ��Ħ���������ֲ���
C�������������ѹǿ���ֲ���
D����λʱ��������nmolCO��ͬʱ����2nmolH2
���ش��������⣺
��1�������Ϊ100ml pH=2��CH3COOH��һԪ��HX����ˮϡ������pH����Һ����Ĺ�ϵ��ͼ��ʾ����Ka(HX) ______ Ka(CH3COOH)���������
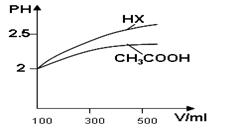
��2��25��ʱ��CH3COOH��CH3COONa�Ļ����Һ�������pH=6������Һ��C(CH3COO?)-c(Na+)=____________mol��L-1(�ȷֵ)��
�Ҵ�������һ������ʳ������ֲ����ά�ӹ��ɵ�ȼ���Ҵ�����ͨ���Ͱ�һ�����������γɵ����������Դ�������ҹ��Ĺ��ұ����Ҵ���������90%����ͨ������10%���Ҵ����Ͷ��ɡ�
��1������ʳ�����ֲ����ά�ɵõ������ǣ�д���������Ƶ��Ҵ��Ļ�ѧ����ʽ: ��
��2���ڳ��³�ѹ�£�1gC2H5OH��ȫȼ������CO2��Һ̬H2Oʱ�ų�29.71 kJ��������ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��3����ͼ��һ���Ҵ�ȼ�ϵ�ع���ʱ��ʾ��ͼ���ҳ��е������缫һ����ʯī�缫��һ�������缫������ʱM��N�����缫�������������٣���ش��������⣺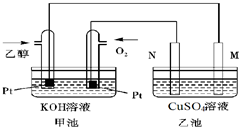
�ټ����Ҵ���Pt�缫�ĵ缫��ӦʽΪ_________________________��
���ڹ��������У��ҳ������缫���ռ�����״����224mL����ʱ���׳����������������������Ϊ mL(��״����)������ʱ�ҳ���Һ���Ϊ200mL�����ҳ�����Һ��pHΪ ��
����Ҫʹ�����ҳص���Һ��ȫ�ָ�����ʼ״̬�������ҳ��м��� (�����)
| A��0.01molCu |
| B��0.01molCuO |
| C��0.01molCu(OH)2 |
| D��0.01molCuCO3 |
F��0.005molCu2(OH)2CO3
��ѧ��Ӧԭ���ڹ�ҵ�����о���ʮ����Ҫ�����塣
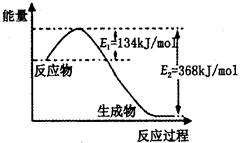
��1����ҵ����������NH3��g����CO2��g������������Ӧ�������أ�������Ӧ�������仯ʾ��ͼ���£�
��NH3��g����CO2��g����Ӧ�������ص��Ȼ�ѧ����ʽΪ ��
��2����֪��ӦFe(s) +CO2(g)  FeO(s) +CO(g) ��H ="a" kJ/mol
FeO(s) +CO(g) ��H ="a" kJ/mol
����ڲ�ͬ�¶��£��÷�Ӧ��ƽ�ⳣ��K���¶ȵı仯���£�
�ٸ÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK= ��a 0���>������<����=��������500�� 2L�ܱ������н��з�Ӧ��Fe��CO2����ʼ����Ϊ4 mol����5 min��ﵽƽ��ʱCO2��ת����Ϊ ������CO��ƽ������v(CO)Ϊ ��
��700�淴Ӧ�ﵽƽ���Ҫʹ��ƽ�������ƶ���������������ʱ�����Բ�ȡ�Ĵ�ʩ��
������ĸ����
| A����С��Ӧ���ݻ� | B������Fe�����ʵ��� |
| C�������¶ȵ�900�� | D��ʹ�ú��ʵĴ��� |
 CH3OH(g)+H2O(g)����ͼ��ʾ�÷�Ӧ���й���������(��λΪkJ��mol��1)�ı仯��
CH3OH(g)+H2O(g)����ͼ��ʾ�÷�Ӧ���й���������(��λΪkJ��mol��1)�ı仯��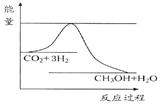
 2NH3��g����H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
2NH3��g����H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���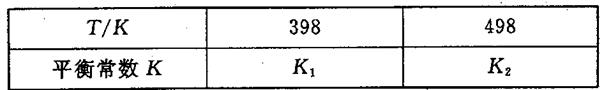
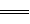 2H2O��l�� ��H����483��6 kJ��mol
2H2O��l�� ��H����483��6 kJ��mol CH3OH(g)����ҵ��������CO����ȼ�ϼ״���
CH3OH(g)����ҵ��������CO����ȼ�ϼ״���
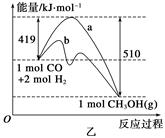
 �������____________��
�������____________��