��Ŀ����
���ǵ����Ϻ����ḻ��һ��Ԫ�أ��䵥�ʼ��������ڹ�ũҵ������������������Ҫ���á�
��1��һ���¶��£���1L�ݻ��㶨���ܱ������г���2 mol N2��8molH2��������Ӧ��10min��ƽ�⣬��ð�����Ũ��Ϊ0��4 mol��L��1����ʱ������ת����Ϊ________��������߰����IJ��ʣ����ݻ�ѧƽ���ƶ�ԭ������������Ľ���______________��д��һ�����ɣ���
��2����ͼ��1mol NO2��g����1mol CO��g����Ӧ����lmol CO2��g����1 mol NO��g�������������仯ʾ��ͼ����д���÷�Ӧ���Ȼ�ѧ����ʽ_____________________��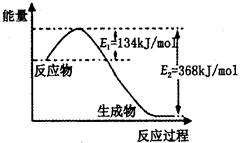
��3�����ݻ��㶨���ܱ������У��������·�Ӧ��N2��g����3H2��g��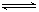 2NH3��g����H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
2NH3��g����H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���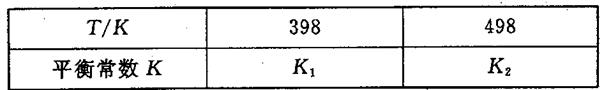
�ٸ÷�Ӧ��ƽ�ⳣ������ʽ��K��_____________��
�����ж�K1__________K2����д��������������������
��NH3��g��ȼ�յķ���ʽΪ��4NH3��g����7O2��g����4NO2��g����6H2O��l������֪��
��2H2��g����O2��g��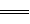 2H2O��l�� ��H����483��6 kJ��mol
2H2O��l�� ��H����483��6 kJ��mol
��N2��g����2O2��g��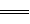 2NO2��g�� ��H����67��8 kJ��mol
2NO2��g�� ��H����67��8 kJ��mol
��N2��g����3H2��g��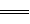 2NH3��g�� ��H����92��0 kJ��mol
2NH3��g�� ��H����92��0 kJ��mol
�����NH3��g����ȼ����________kJ��mol��
��1��10%������Ӧ���Ũ�ȡ����¡�����ѹǿ�ȣ�
��2��NO2��g��+CO��g��=CO2��g��+NO��g����H=-234 kJ��mol��
��3����K=c2(NH3)/ c(N2) c3(H2)���ڣ�����282.8
���������������1����������֪��ƽ��ʱ���������ʵ���Ϊ0.4mol������N2��g����3H2��g��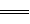 2NH3��g��֪��ת���ĵ��������ʵ���Ϊ0.2mol��������ת����Ϊ10%��������߰����IJ��ʣ����ݻ�ѧƽ���ƶ�ԭ������������Ľ��飺����Ӧ���Ũ�ȡ����¡�����ѹǿ�ȣ���2�������ͼ��֪���÷�Ӧ���ʱ��H=E1��E2=134KJ/mol��368KJ/mol=��234KJ/mol���Ȼ�ѧ����ʽΪNO2��g��+CO��g��=CO2��g��+NO��g����H=��234 kJ��mol����3���ٸ��ݻ�ѧƽ�ⳣ���Ķ���д����K=c2(NH3)/ c(N2) c3(H2)���ںϳɰ��ķ�ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�������ƶ���ƽ�ⳣ����С������K1��K2���۸��ݸ�˹���ɣ��١�3+�ڡ�2���ۡ�2�ã�4NH3��g����7O2��g����4NO2��g����6H2O��l������H=��1131.2 kJ��mol�����ȼ���ȵĶ���֪��NH3��g����ȼ����282.8kJ��mol��
2NH3��g��֪��ת���ĵ��������ʵ���Ϊ0.2mol��������ת����Ϊ10%��������߰����IJ��ʣ����ݻ�ѧƽ���ƶ�ԭ������������Ľ��飺����Ӧ���Ũ�ȡ����¡�����ѹǿ�ȣ���2�������ͼ��֪���÷�Ӧ���ʱ��H=E1��E2=134KJ/mol��368KJ/mol=��234KJ/mol���Ȼ�ѧ����ʽΪNO2��g��+CO��g��=CO2��g��+NO��g����H=��234 kJ��mol����3���ٸ��ݻ�ѧƽ�ⳣ���Ķ���д����K=c2(NH3)/ c(N2) c3(H2)���ںϳɰ��ķ�ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�������ƶ���ƽ�ⳣ����С������K1��K2���۸��ݸ�˹���ɣ��١�3+�ڡ�2���ۡ�2�ã�4NH3��g����7O2��g����4NO2��g����6H2O��l������H=��1131.2 kJ��mol�����ȼ���ȵĶ���֪��NH3��g����ȼ����282.8kJ��mol��
���㣺���黯ѧƽ����㡢ƽ���ƶ�ԭ������ѧƽ�ⳣ����Ӱ�����ء��Ȼ�ѧ����ʽ����д��ȼ���ȵĸ��

(1)�״���һ����Ҫ�Ļ�����Ʒ�������ü״��������Ʊ���ȩ����ȩ����̬�״�ת����������ϵ��ͼ��ʾ��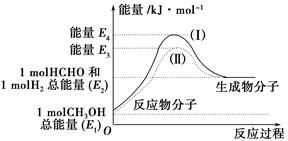
��Ӧ�����е�������ϵ
�ټ״�������ת��Ϊ��ȩ�ķ�Ӧ��________(����ȡ����ȡ�)��Ӧ��
�ڹ��̢�����̢�ķ�Ӧ���Ƿ���ͬ��____________ԭ����__________________________________��
��д���״�������ת��Ϊ��ȩ���Ȼ�ѧ��Ӧ����ʽ________________________________��
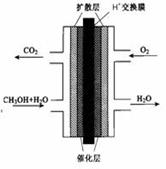
(2)��֪����CH3OH(g)��H2O(g)=CO2(g)��3H2(g)����H����49.0 kJ��mol��1
��CH3OH(g)��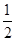 O2(g)=CO2(g)��2H2(g)����H����192.9 kJ��mol��1
O2(g)=CO2(g)��2H2(g)����H����192.9 kJ��mol��1
����˵����ȷ����________��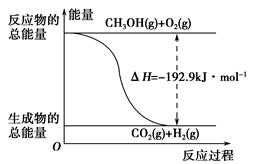
| A��CH3OHת���H2�Ĺ���һ��Ҫ�������� |
| B���ٷ�Ӧ�У���Ӧ�������������������������� |
C�����ݢ���֪��Ӧ��CH3OH(l)��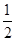 O2(g)=CO2(g)��2H2(g)�Ħ�H����192.9 kJ��mol��1 O2(g)=CO2(g)��2H2(g)�Ħ�H����192.9 kJ��mol��1 |
| D����Ӧ�ڵ������仯��ͼ��ʾ |
��1����֪��Ӧ�����������л�ѧ������ʱ�������仯������ʾ��
H2(g)��Cl2(g)��2HCl(g) ��H����184kJ/mol
4HCl (g)��O2(g)��2Cl2 (g) ��2H2O (g) ��H����115.6kJ/mol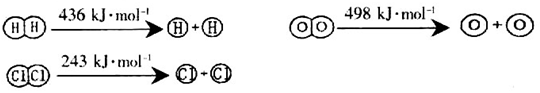
��H2��O2��Ӧ������̬ˮ���Ȼ�ѧ����ʽΪ_________________________________��
�ڶϿ�1mol H��O����������ԼΪ_________________________kJ��
��2����֪ij��Ӧ��ƽ�ⳣ������ʽΪ��K�� ,������Ӧ�Ļ�ѧ����ʽΪ________________��
,������Ӧ�Ļ�ѧ����ʽΪ________________��
��3����֪��ӦN2(g)��3H2(g) 2NH3(g) ��H��0��400��ʱK��0.5������������0.5L���ܱ������н��и÷�Ӧ��һ��ʱ����N2��H2��NH3�����ʵ����ֱ�Ϊ2mol��1mol��2mol�����ʱ��Ӧ��(N2)��______��(N2)�棨�����������������������ʹ�ø÷�Ӧ�Ļ�ѧ��Ӧ���ʼӿ죬ͬʱʹƽ��ʱNH3������ٷ������ӣ��ɲ�ȡ�Ĵ�ʩ��_______������ţ���
2NH3(g) ��H��0��400��ʱK��0.5������������0.5L���ܱ������н��и÷�Ӧ��һ��ʱ����N2��H2��NH3�����ʵ����ֱ�Ϊ2mol��1mol��2mol�����ʱ��Ӧ��(N2)��______��(N2)�棨�����������������������ʹ�ø÷�Ӧ�Ļ�ѧ��Ӧ���ʼӿ죬ͬʱʹƽ��ʱNH3������ٷ������ӣ��ɲ�ȡ�Ĵ�ʩ��_______������ţ���
A.��С�������ѹǿ B.�����¶�
C.�Ӵ��� D.ʹ����Һ������
��4����һ��������ܱ������н������»�ѧ��Ӧ��A(g)��3B(g) 2C(g)��D(s) ��H���仯ѧƽ�ⳣ��K��T�Ĺ�ϵ���±���
2C(g)��D(s) ��H���仯ѧƽ�ⳣ��K��T�Ĺ�ϵ���±���
| T/K | 300 | 400 | 500 | ���� |
| K/(mol��L��1)2 | 4��106 | 8��107 | 1.2��109 | ���� |
����һ�������£����жϸ÷�Ӧһ���ﻯѧƽ��״̬����______������ţ���
A.3 ��(B)����2��(C)�� B.A��B��ת�������
C.������ѹǿ���ֲ��� D.���������ܶȱ��ֲ���
�����γɶ����������NO��NO2��N2O4�ȡ���֪NO2��N2O4�Ľṹʽ�ֱ���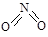 ��
��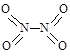 ��ʵ����N��N������Ϊ167kJ��mol��1�� NO2�е�������ƽ������Ϊ466 kJ��mol��1��N2O4�е�������ƽ������Ϊ438.5 kJ��mol��1��
��ʵ����N��N������Ϊ167kJ��mol��1�� NO2�е�������ƽ������Ϊ466 kJ��mol��1��N2O4�е�������ƽ������Ϊ438.5 kJ��mol��1��
��1��д��N2O4ת��ΪNO2���Ȼ�ѧ����ʽ��
��2���Է�ӦN2O4(g) 2NO2(g)�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ����
2NO2(g)�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ���� 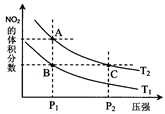
A��A��C����ķ�Ӧ���ʣ�A��C
B��B��C����������ƽ����Է���������B��C
C��A��C�����������ɫ��A�Cdz
D����״̬B��״̬A�������ü��ȵķ���
��3����100��ʱ����0.40mol��NO2�������2 L��յ��ܱ������У�ÿ��һ��ʱ��ͶԸ������ڵ����ʽ��з������õ����±����ݣ�
| ʱ�䣨s�� | 0 | 20 | 40 | 60 | 80 |
| n(NO2)/mol | 0.40 | n1 | 0.26 | n3 | n4 |
| n(N2O4)/mol | 0.00 | 0.050 | n2 | 0.080 | 0.080 |
�������������£��ӷ�Ӧ��ʼֱ��20 sʱ������������ƽ����Ӧ����Ϊ
��n3 n4���>������<����=�������÷�Ӧ��ƽ�ⳣ��K��ֵΪ �������¶Ⱥ�Ӧ2NO2
 N2O4��ƽ�ⳣ��K�� �����������С�����䡱����
N2O4��ƽ�ⳣ��K�� �����������С�����䡱������������ͬ����������������������N2O4���壬Ҫ�ﵽ����ͬ����ƽ��״̬��N2O4����ʼŨ����_____________mol��L��1��
��1��8gҺ̬��CH3OH����������ȫȼ�գ����ɶ�����̼�����Һ̬ˮʱ�ͷų�Q kJ����������д��Һ̬CH3OHȼ���ȵ��Ȼ�ѧ����ʽ ��
��2���ڻ�ѧ��Ӧ�����У��ƻ��ɻ�ѧ����Ҫ�����������γ��»�ѧ���ֻ��ͷ�������
| ��ѧ�� | H��H | N��H | N��N |
| ����/kJ��mol��1 | 436 | 391 | 945 |
��֪��ӦN2��3H2=2NH3����H��a KJ/mol��
�Ը��ݱ������м������ݼ���a����ֵΪ�� ��
��3����֪��C(s��ʯī)��O2(g)=CO2(g) ��H1����393.5 kJ/mol
2H2(g)��O2(g)=2H2O(l) ��H2����571.6 kJ/mol
2C2H2(g)��5O2(g)=4CO2(g)��2H2O(l) ��H3����2599 kJ/mol
���ݸ�˹���ɣ���C(s��ʯī)��H2(g)����1 mol C2H2(g)��Ӧ���Ȼ�ѧ����ʽ�� ��
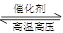 2NH3(g) ��H����92.4 kJ��mol��1
2NH3(g) ��H����92.4 kJ��mol��1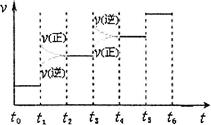
 2PbSO4��2H2O����ش��������⣨�������⡢����������ԭ����
2PbSO4��2H2O����ش��������⣨�������⡢����������ԭ���� 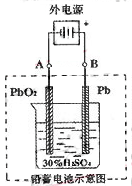
 2CO2(g)+ N2(g)����H��0
2CO2(g)+ N2(g)����H��0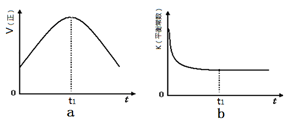
 N2O4(g) ��H����56.9 kJ/mol
N2O4(g) ��H����56.9 kJ/mol