��Ŀ����
�Ҵ�������һ������ʳ������ֲ����ά�ӹ��ɵ�ȼ���Ҵ�����ͨ���Ͱ�һ�����������γɵ����������Դ�������ҹ��Ĺ��ұ����Ҵ���������90%����ͨ������10%���Ҵ����Ͷ��ɡ�
��1������ʳ�����ֲ����ά�ɵõ������ǣ�д���������Ƶ��Ҵ��Ļ�ѧ����ʽ: ��
��2���ڳ��³�ѹ�£�1gC2H5OH��ȫȼ������CO2��Һ̬H2Oʱ�ų�29.71 kJ��������ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��3����ͼ��һ���Ҵ�ȼ�ϵ�ع���ʱ��ʾ��ͼ���ҳ��е������缫һ����ʯī�缫��һ�������缫������ʱM��N�����缫�������������٣���ش��������⣺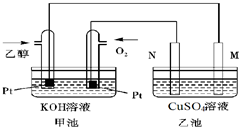
�ټ����Ҵ���Pt�缫�ĵ缫��ӦʽΪ_________________________��
���ڹ��������У��ҳ������缫���ռ�����״����224mL����ʱ���׳����������������������Ϊ mL(��״����)������ʱ�ҳ���Һ���Ϊ200mL�����ҳ�����Һ��pHΪ ��
����Ҫʹ�����ҳص���Һ��ȫ�ָ�����ʼ״̬�������ҳ��м��� (�����)
| A��0.01molCu |
| B��0.01molCuO |
| C��0.01molCu(OH)2 |
| D��0.01molCuCO3 |
F��0.005molCu2(OH)2CO3
��1��C6H12O6 2C2H5OH+2CO2����2�֣�
2C2H5OH+2CO2����2�֣�
��2��C2H5OH(l)+3O2 (g) =2CO2 (g)+3H2O(l)???H="��1366.7" kJ/mol��2�֣�
��3����C2H5OH-12e-+16 OH��= 2CO32-+11H2O��2�֣�
��224��2�֣���1��2�֣�
��C��2�֣���ѡ���÷֣�
���������������3�����ҳ��е������缫һ����ʯī�缫��һ�������缫������ʱM��N�����缫�������������٣�������������������������缫��Ӧ����ô��M��N�����缫�Ϸ����ĵ缫��ӦΪ��
M����������Cu2����2e����Cu 2H����2e����H2��
N����������4OH�D�D4e����2H2O��O2��
�ҳ������缫���ռ�����״����224mL����ʱ��ת�Ƶĵ������ʵ���Ϊ��0.04mol����ô����Ҳת��0.04mol�ĵ��ӣ���˼׳����������������������Ϊ224mL���ҳ�����Ϊֻ��0.02mol��������������������ô��Һ�л�Ӧ��ʣ��0.02mol�������ӣ���ô������Һ��������Ũ��Ϊ��c(H��)��0.1mol/L�������ҳ�����Һ��pHΪ1
���㣺����绯ѧ���й�֪ʶ��

 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д���10�֣���ҵ���Ʊ�BaCl2�Ĺ�������ͼ��ͼ��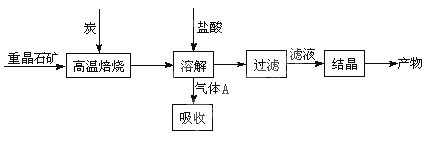
ij�о�С����ʵ�������ؾ�ʯ����Ҫ�ɷ�BaSO4���Թ�ҵ���̽���ģ��ʵ�顣����
�ϵã�
BaSO4(s) + 4C(s)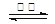 4CO(g) + BaS(s) ��H1 = +571��2 kJ��mol-1 ��
4CO(g) + BaS(s) ��H1 = +571��2 kJ��mol-1 ��
BaSO4(s) + 2C(s)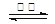 2CO2(g) + BaS(s) ��H2= +226��2 kJ��mol-1 ��
2CO2(g) + BaS(s) ��H2= +226��2 kJ��mol-1 ��
��1�����Ʊ�BaCl2�Ĺ�������ͼ������A�ù���NaOH��Һ���գ��õ����ơ�Na2Sˮ������ӷ���ʽΪ ��
�ڳ����£���ͬŨ�ȵ�Na2S��NaHS��Һ�У�����˵����ȷ���� ������ĸ����
| A��Na2S��Һ��pH��NaHS��ҺpHС |
| B������Һ�к��е��������ͬ |
| C������Һ�е���ͬ���ͬŨ�ȵ����ᣬ��������������� |
| D������Һ�м���NaOH���壬c(S2-)������ |
��2����BaCl2��Һ�м���AgNO3��KBr�������ֳ�������ʱ��
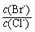 = �� ����֪��Ksp(AgBr)=5��4��10-13��Ksp(AgCl)=2��0��10-10��
= �� ����֪��Ksp(AgBr)=5��4��10-13��Ksp(AgCl)=2��0��10-10����3����ӦC(s) + CO2(g)
 2CO(g)�Ħ�H =
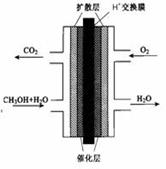
2CO(g)�Ħ�H = ��4��ʵ�������б�����������̿��ͬʱ��Ҫͨ���������Ŀ���ǣ� ��ֻҪ���һ������
��1��8gҺ̬��CH3OH����������ȫȼ�գ����ɶ�����̼�����Һ̬ˮʱ�ͷų�Q kJ����������д��Һ̬CH3OHȼ���ȵ��Ȼ�ѧ����ʽ ��
��2���ڻ�ѧ��Ӧ�����У��ƻ��ɻ�ѧ����Ҫ�����������γ��»�ѧ���ֻ��ͷ�������
| ��ѧ�� | H��H | N��H | N��N |
| ����/kJ��mol��1 | 436 | 391 | 945 |
��֪��ӦN2��3H2=2NH3����H��a KJ/mol��
�Ը��ݱ������м������ݼ���a����ֵΪ�� ��
��3����֪��C(s��ʯī)��O2(g)=CO2(g) ��H1����393.5 kJ/mol
2H2(g)��O2(g)=2H2O(l) ��H2����571.6 kJ/mol
2C2H2(g)��5O2(g)=4CO2(g)��2H2O(l) ��H3����2599 kJ/mol
���ݸ�˹���ɣ���C(s��ʯī)��H2(g)����1 mol C2H2(g)��Ӧ���Ȼ�ѧ����ʽ�� ��
 2PbSO4��2H2O����ش��������⣨�������⡢����������ԭ����
2PbSO4��2H2O����ش��������⣨�������⡢����������ԭ���� 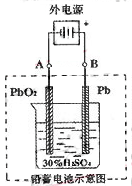
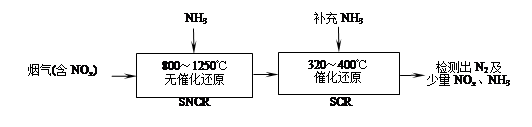
 2CO2��N2�ܹ��Է����У���÷�Ӧ�Ħ�H 0���������������
2CO2��N2�ܹ��Է����У���÷�Ӧ�Ħ�H 0���������������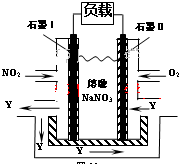
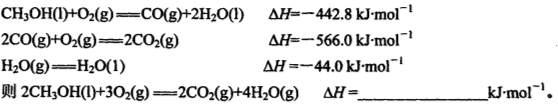
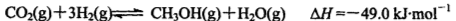
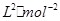 mol;
mol;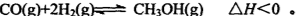

 ���>������<����=����
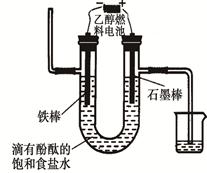
���>������<����=����
 2NH3(g) ��H����92 kJ/mol
2NH3(g) ��H����92 kJ/mol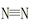 ����H��H�����ֱ������յ�����Ϊ946 kJ��436 kJ����Ͽ�1molN��H�����յ�����Ϊ kJ��
����H��H�����ֱ������յ�����Ϊ946 kJ��436 kJ����Ͽ�1molN��H�����յ�����Ϊ kJ��