��Ŀ����
A��B��C��D��E��F���ڶ���������Ԫ�أ�A��ԭ�Ӱ뾶�ڶ����������BԪ�ص�ԭ������������Ϊm������������Ϊn��CԪ�ص�ԭ��L�������Ϊm+n��M�������Ϊm-n��DԪ����CԪ��ͬ���壬EԪ��ԭ����BԪ��ԭ�ӵĺ��������֮��Ϊ2��1��F��һ��ԭ���У���������������֮��Ϊ�㣮��l��DԪ�ػ�̬ԭ�ӵĵ����Ų�ʽ______��DԪ����BԪ�ص縺�ԣ�B______D�������=��������
��2��A��B��EԪ���γɵļ����ӵİ뾶�ɴ�С��˳��Ϊ______�������ӷ��ű�ʾ����Ԫ��B��C��E�γɵij������ʣ��۷е��ɸߵ��͵�˳����______�������ƣ���
��3����D��FԪ�ؿ��Թ��ɵ���Ļ�����W��ȡ32g W��ȫȼ������Һ̬ˮ���ɷų�1780.6kJ����������Wȼ�յ��Ȼ�ѧ����ʽΪ______��
��4���������ȼ�ϵ���DZ����������ս�ԵĿ���֮һ������������һ��ȼ�ϵ�أ�һ���缫ͨ�븻��B2���ʵ����������
DB2����һ�缫ͨ��W���壬��صĵ���������ڵ�K2CO3����صĸ�����ӦʽΪ______����ع���ʱ����������CO32-��______���ƶ���
��5����DB����2.24L����״�������������в����õ��ȼ���õ��IJ���ͨ�������� Na2O2�У���ַ�Ӧ��Na2O2����______g��
���𰸡�������A��B��C��D��E��F���ڶ���������Ԫ�أ�A��ԭ�Ӱ뾶�ڶ������������AΪNaԪ�أ�
CԪ�ص�ԭ��L�������Ϊm+n��M�������Ϊm-n������m+n=8��BԪ�ص�ԭ������������Ϊm������������Ϊn������m+n=8������n��8����BԪ�صĴ����ΪK�㣬��n=2������m=6����CΪSiԪ�أ�BΪOԪ�أ�
DԪ����CԪ��ͬ���壬����DΪCԪ�أ�
EԪ��ԭ����BԪ��ԭ�ӵĺ��������֮��Ϊ2��1������EΪSԪ�أ�
F��һ��ԭ���У���������������֮��Ϊ�㣬��ԭ�������ӣ�����FΪHԪ�أ�
����⣺�ɷ�����֪��AΪNaԪ�أ�BΪOԪ�أ�CΪSiԪ�أ�DΪCԪ�أ�EΪSԪ�أ�FΪHԪ�أ�
��1��DΪCԪ�أ�������Ϊ6��ԭ�Ӻ��������Ϊ6����̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p2��
DΪCԪ�ء�BΪOԪ�أ�ͬ����������ҵ縺�Գ��������ƣ����Ե縺��O��C����B��D��
�ʴ�Ϊ��1s22s22p2������
��2��A��B��EԪ���γɵļ����ӷֱ���Na+��O2-��S2-��Na+��O2-���ӵĺ�������Ų���ͬ���˵����Խ�����Ӱ뾶ԽС���������Ӱ뾶O2-��Na+��S2-��O2-��������������ͬ�����Ӳ�Խ�࣬���Ӱ뾶Խ���������Ӱ뾶S2-��O2-���������Ӱ뾶S2-��O2-��Na+��
Ԫ��B��C��E�γɵij������ʣ��ֱ�Ϊ�赥�ʡ����ʡ��������赥��Ϊԭ�Ӿ��壬����Ϊ���Ӿ��壬�������γɷ��Ӿ��峣����Ϊ���壬�����۷е�裾��ǣ�������
�ʴ�Ϊ��S2-��O2-��Na+���裾��ǣ�������
��3����D��FԪ�ؿ��Թ��ɵ���Ļ�����WΪCH4��32gCH4�����ʵ���Ϊ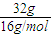 =2mol����ȫȼ������Һ̬ˮ���ų�1780.6kJ����������CH4ȼ�յ��Ȼ�ѧ����ʽΪCH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890.3kJ?mol-1
=2mol����ȫȼ������Һ̬ˮ���ų�1780.6kJ����������CH4ȼ�յ��Ȼ�ѧ����ʽΪCH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890.3kJ?mol-1
��
�ʴ�Ϊ��CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890.3kJ?mol-1��
��4��ԭ��ر���ΪCH4+2O2=CO2+2H2O��ԭ��ظ�������������Ӧ������CH4�ڸ����ŵ磬���ɶ�����̼��ˮ���缫��ӦʽΪCH4-8e-+4CO32-=5CO2+2H2O����ع���ʱ����������CO32-���ƶ���
�ʴ�Ϊ��CH4-8e-+4CO32-=5CO2+2H2O��������
��5��DB����ΪCO��2.24L����״����CO�����ʵ���Ϊ0.1mol������Ϊ0.1mol×28g/mol=2.8g�����������в����õ��ȼ���ɶ�����̼��������̼��������Ʒ�Ӧ��2CO+O2=2CO2��2Na2O2+2CO2=2Na2CO3+O2���ɷ���ʽ��֪������������ΪCO������Ϊ2.8g��
�ʴ�Ϊ��2.8g��
��������ԭ�ӽṹ��λ�ù�ϵΪ���壬���黯ѧ�������ṹ�����ʡ��뾶�Ƚϵȡ�ԭ��ء���ѧ����ȣ��ۺ��Խϴ��Ѷ��еȣ��ƶ�Ԫ���ǽ���ؼ����Ƕ���ѧ֪ʶ���ۺ����ã�ע�����֪ʶ���������գ�
CԪ�ص�ԭ��L�������Ϊm+n��M�������Ϊm-n������m+n=8��BԪ�ص�ԭ������������Ϊm������������Ϊn������m+n=8������n��8����BԪ�صĴ����ΪK�㣬��n=2������m=6����CΪSiԪ�أ�BΪOԪ�أ�
DԪ����CԪ��ͬ���壬����DΪCԪ�أ�
EԪ��ԭ����BԪ��ԭ�ӵĺ��������֮��Ϊ2��1������EΪSԪ�أ�
F��һ��ԭ���У���������������֮��Ϊ�㣬��ԭ�������ӣ�����FΪHԪ�أ�
����⣺�ɷ�����֪��AΪNaԪ�أ�BΪOԪ�أ�CΪSiԪ�أ�DΪCԪ�أ�EΪSԪ�أ�FΪHԪ�أ�
��1��DΪCԪ�أ�������Ϊ6��ԭ�Ӻ��������Ϊ6����̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p2��
DΪCԪ�ء�BΪOԪ�أ�ͬ����������ҵ縺�Գ��������ƣ����Ե縺��O��C����B��D��
�ʴ�Ϊ��1s22s22p2������
��2��A��B��EԪ���γɵļ����ӷֱ���Na+��O2-��S2-��Na+��O2-���ӵĺ�������Ų���ͬ���˵����Խ�����Ӱ뾶ԽС���������Ӱ뾶O2-��Na+��S2-��O2-��������������ͬ�����Ӳ�Խ�࣬���Ӱ뾶Խ���������Ӱ뾶S2-��O2-���������Ӱ뾶S2-��O2-��Na+��
Ԫ��B��C��E�γɵij������ʣ��ֱ�Ϊ�赥�ʡ����ʡ��������赥��Ϊԭ�Ӿ��壬����Ϊ���Ӿ��壬�������γɷ��Ӿ��峣����Ϊ���壬�����۷е�裾��ǣ�������
�ʴ�Ϊ��S2-��O2-��Na+���裾��ǣ�������
��3����D��FԪ�ؿ��Թ��ɵ���Ļ�����WΪCH4��32gCH4�����ʵ���Ϊ
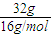 =2mol����ȫȼ������Һ̬ˮ���ų�1780.6kJ����������CH4ȼ�յ��Ȼ�ѧ����ʽΪCH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890.3kJ?mol-1
=2mol����ȫȼ������Һ̬ˮ���ų�1780.6kJ����������CH4ȼ�յ��Ȼ�ѧ����ʽΪCH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890.3kJ?mol-1��
�ʴ�Ϊ��CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890.3kJ?mol-1��
��4��ԭ��ر���ΪCH4+2O2=CO2+2H2O��ԭ��ظ�������������Ӧ������CH4�ڸ����ŵ磬���ɶ�����̼��ˮ���缫��ӦʽΪCH4-8e-+4CO32-=5CO2+2H2O����ع���ʱ����������CO32-���ƶ���
�ʴ�Ϊ��CH4-8e-+4CO32-=5CO2+2H2O��������
��5��DB����ΪCO��2.24L����״����CO�����ʵ���Ϊ0.1mol������Ϊ0.1mol×28g/mol=2.8g�����������в����õ��ȼ���ɶ�����̼��������̼��������Ʒ�Ӧ��2CO+O2=2CO2��2Na2O2+2CO2=2Na2CO3+O2���ɷ���ʽ��֪������������ΪCO������Ϊ2.8g��
�ʴ�Ϊ��2.8g��
��������ԭ�ӽṹ��λ�ù�ϵΪ���壬���黯ѧ�������ṹ�����ʡ��뾶�Ƚϵȡ�ԭ��ء���ѧ����ȣ��ۺ��Խϴ��Ѷ��еȣ��ƶ�Ԫ���ǽ���ؼ����Ƕ���ѧ֪ʶ���ۺ����ã�ע�����֪ʶ���������գ�

��ϰ��ϵ�д�
�����Ŀ
 [��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ�
[��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ�