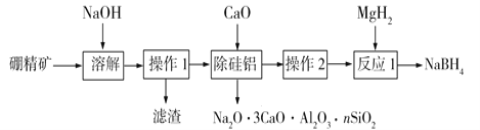
��Ŀ����
����Ŀ��ij��ѧ��ȤС��Ե������Һ�����µĹ����ܽᣨ���ڳ����£���������ȷ����
A. pH=3��ǿ����Һ1mL����ˮϡ����100mL����ҺpH����2����λ
B. 1L 0.50mol��L-1NH4Cl ��Һ��2L 0.25mol��L-1NH4Cl ��Һ��NH4+���ʵ������ߴ�
C. pH=8.3��NaHCO3��Һ��c(Na+)��c(HCO3��)��c(CO32-)��c(H2CO3)
D. pH=4��Ũ�Ⱦ�Ϊ0.1mol��L-1��CH3COOH��CH3COONa�����Һ�У�c(CH3COO��)��c(CH3COOH)=2[c(H��)��c(OH��)]=![]()
���𰸡�D
��������
���ˮϡ�ͣ�������Ũ�Ƚ��ͣ�pH���ߣ�笠�������ˮ��Һ�лᷢ��ˮ�⣬NH4ClŨ��ԽС��NH4��ˮ��̶�Խ��NaHCO3��Һ�Լ��ԣ�̼��������ӵ�ˮ��̶ȴ��ڵ���̶ȣ�ˮ������̼�ᣬ��������̼������ӣ��ݴ��жϣ������£����ҺpH=4��![]() ������Kw��֪��
������Kw��֪��![]() ���ɵ���غ��֪��c(CH3COO��)��c(Na��)=c(H��)-c(OH��)���������غ㣺2c(Na��)= c��CH3COOH����c(CH3COO��)���ݴ˷�����
���ɵ���غ��֪��c(CH3COO��)��c(Na��)=c(H��)-c(OH��)���������غ㣺2c(Na��)= c��CH3COOH����c(CH3COO��)���ݴ˷�����
A��pH=3��ǿ���ˮϡ�ͣ�pH���ߣ��ʲ��������⣻
B����ͬŨ�ȵ�ͬ����Һ��Ũ��ԽС��ˮ��̶�Խ��![]() NH4Cl��Һ��NH4��ˮ��̶ȴʲ��������⣻
NH4Cl��Һ��NH4��ˮ��̶ȴʲ��������⣻
C��NaHCO3��Һ�Լ��ԣ�˵��HCO3��ˮ��̶ȴ��ڵ���̶ȣ�������Ũ�ȹ�ϵΪc(Na��)>c(HCO3��)>c(H2CO3)>c(CO32��)���ʲ��������⣻
D�������£����ҺpH=4![]() ������K��֪��
������K��֪��![]() ���ɵ���غ��֪��c(CH3COO��)��c(Na��)=c(H��)��c(OH��)���������غ㣺
���ɵ���غ��֪��c(CH3COO��)��c(Na��)=c(H��)��c(OH��)���������غ㣺
2c(Na��)= c��CH3COOH����c(CH3COO��)�������ã�c(CH3COO��)��c(CH3COOH)=2[c(H��)��c(OH��)]=![]() ���ʷ������⣻
���ʷ������⣻
��ѡD��

����Ŀ�������Ǹ�ˮ����[Cr(CH3COO)2]22H2O������Ϣ���£�
�������� | ��ѧ���� |
����ɫ���壬�����Ҵ�����������ˮ������(�ӷ����л��ܼ�) | ����ǿ��ԭ�ԣ��ױ����� |
�Ʊ�ԭ����2Cr2��(aq)��4CH3COO��(aq)��2H2O(l)��[Cr(CH3COO)2]22H2O(s)��
ij��ȤС�����ʵ���Ʊ�[Cr(CH3COO)2]22H2O(s)��

�ش��������⣺
(1)����A��������_____��
(2)���װ��B�����ԵIJ���������_____��
(3)��������ر�K1����K2����װ��B�еĵ��ܳ��Һ������һ��ʱ�䣬Ŀ����____����Ӧ��ʼ��װ��B�п�������������Һ������ɫ(Cr3��)��Ϊ����ɫ(Cr2��)���������ݲ�����д��װ��B�з����ķ��û���Ӧ�����ӷ���ʽ��_____��
(4)�������ķų����ʽϿ�ʱ��Ϊ��ʹװ��B����Һ����װ��C�У��˲�ȡ�IJ�����_____��װ��D�е��ܿ�ˮ���Ŀ����_____��
(5)��װ��C�����ò�Ʒ�ᴿ�����������Ϊ���ˡ�ȥ��ˮϴ�ӡ�����ϴ�ӡ�����Ҵ���ˮ��������ϴ�ӵ��ŵ���______��
(6)�ⶨ��Ʒ���ȣ�ȡag��Ʒ��������ˮ��ͨ��������������ַ�Ӧ�����������������Һ�����ˡ�ϴ�ӡ����ա����ء���Cr2O3����Ϊmg(�������ʲ����뷴Ӧ)��[Cr(CH3COO)2]22H2O(s)��Ħ������ΪMg��mol��1����ò�Ʒ����Ϊ____ %��(�ú�a��m��M�Ĵ���ʽ��ʾ)