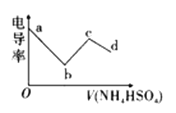
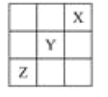
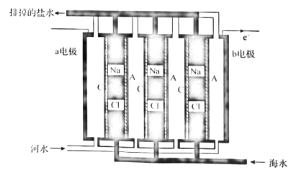
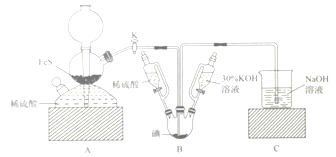
��Ŀ����
����Ŀ��A��B��C��D��E�������ʵ���ɫ��Ӧ���ʻ�ɫ��A��B��ˮ��Ӧ��������ų���A��ˮ��Ӧ�ų���������л�ԭ�ԣ�B��ˮ��Ӧ�ų���������������ԣ�ͬʱ������C����Һ��C��������CO2��Ӧ����D��D��Һ�������CO2��Ӧ����E��E����������D��
��1��д���������ʵĻ�ѧʽ��B____________��E_____________��
��2��д��A��C�Ļ�ѧ����ʽ�����������ת�Ƶķ������Ŀ��_______________
��3��д��C��D�����ӷ���ʽ��________________________________��
��4��д��E��D�Ļ�ѧ����ʽ��________________________________��
���𰸡�Na2O2 NaHCO3 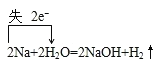 2OH-+CO2=CO32-+H2O 2NaHCO3
2OH-+CO2=CO32-+H2O 2NaHCO3![]() Na2CO3+CO2��+H2O
Na2CO3+CO2��+H2O
��������
A��B��C��D��E�������ʵ���ɫ��Ӧ���ʻ�ɫ������NaԪ�أ�A��B��ˮ��Ӧ��������ų���A��ˮ��Ӧ�ų���������л�ԭ�ԣ�B��ˮ��Ӧ�ų���������������ԣ���AΪNa��BΪNa2O2��ͬʱ��������ҺC����CΪNaOH��C��������ɫ��ζ��CO2���巴Ӧ����D��D��Һ�������CO2���巴Ӧ����E��E�����ܹ�����D����DΪNa2CO3��EΪNaHCO3��������ʵ����ʼ���ѧ���������
A��B��C��D��E�������ʵ���ɫ��Ӧ���ʻ�ɫ������NaԪ�أ�A��B��ˮ��Ӧ��������ų���A��ˮ��Ӧ�ų���������л�ԭ�ԣ�B��ˮ��Ӧ�ų���������������ԣ���AΪNa��BΪNa2O2��ͬʱ��������ҺC����CΪNaOH��C��������ɫ��ζ��CO2���巴Ӧ����D��D��Һ�������CO2���巴Ӧ����E��E�����ܹ�����D����DΪNa2CO3��EΪNaHCO3��
��1��������������֪��BΪNa2O2��EΪNaHCO3���ʴ�Ϊ��Na2O2��NaHCO3��
��2��AΪNa��CΪNaOH����A��C�Ļ�ѧ����ʽ�����������ת�Ƶķ������ĿΪ��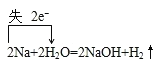 ��
��
��3��C��Һ��CO2��Ӧ����D�����ӷ���ʽΪ��2OH-+CO2=CO32-+H2O���ʴ�Ϊ��2OH-+CO2=CO32-+H2O��
��4��EΪNaHCO3��DΪNa2CO3����E��D�Ļ�ѧ����ʽΪ��2NaHCO3![]() Na2CO3+CO2��+H2O���ʴ�Ϊ��2NaHCO3
Na2CO3+CO2��+H2O���ʴ�Ϊ��2NaHCO3![]() Na2CO3+CO2��+H2O��
Na2CO3+CO2��+H2O��