��Ŀ����
����˵����ȷ���ǣ�������
| A��0.1mol/L NaHCO3��Һ�У�c��HCO3-��+c��CO32-��+c��OH-����c��Na+��+c��H+��+c��H2CO3�� |
| B�������£�5mL 0.02mol/L HCl��Һ��5mL 0.02mol/L Ba��OH��2��Һ��ϣ�����ַ�Ӧ�������Һ���Ϊ10mL������ҺpH=12 |
| C����ͬ������pH=5�Ģ�NH4Cl��Һ ��CH3COOH��Һ ��ϡ���ᣬ������Һ����ˮ���������c��H+�����٣��ڣ��� |
| D�������£���ϡNaOH��Һ��CH3COOH��Һ��ϣ����ܳ���pH��7����c��OH-����c��Na+����c��H+����c��CH3COO-������� |
���㣺����Ũ�ȴ�С�ıȽ�
ר�⣺����ƽ������Һ��pHר��,�����ˮ��ר��
������A��̼��������Һ�У�̼��������Ӳ���ˮ�⣬���c��Na+����c��HCO3-�����ٸ��ݵ���غ㡢�����غ�ɵâ�c��H2CO3��+c��H+��=c��CO32-��+c��OH-���������ݢ٢��ж�����Ũ�ȴ�С��
B���������������������Һ�������ӡ����������ӵ����ʵ����ж���Һ������ԣ�Ȼ���������Һ��pH��
C������Һ������Һ������ˮ�ĵ��룬�ܹ�ˮ�������Һ�ٽ���ˮ�ĵ��룬�ݴ˽����жϣ�
D����ϡNaOH��Һ��CH3COOH��Һ��������ϣ������ܳ���c��OH-����c��Na+����c��H+����c��CH3COO-����
B���������������������Һ�������ӡ����������ӵ����ʵ����ж���Һ������ԣ�Ȼ���������Һ��pH��
C������Һ������Һ������ˮ�ĵ��룬�ܹ�ˮ�������Һ�ٽ���ˮ�ĵ��룬�ݴ˽����жϣ�
D����ϡNaOH��Һ��CH3COOH��Һ��������ϣ������ܳ���c��OH-����c��Na+����c��H+����c��CH3COO-����
���
�⣺A�����ݵ���غ�ɵã�c��Na+��+c��H+��=c��HCO3-��+2c��CO32-��+c��OH-������Һ���������غ㣬��c��Na+��=c��HCO3-��+c��CO32-��+c��H2CO3�����������Ӵ��˵���غ�ɵã�c��H2CO3��+c��H+��=c��CO32-��+c��OH-����������Һ��̼��������Ӳ���ˮ�⣬��c��Na+����c��HCO3-������c��HCO3-��+c��CO32-��+c��OH-����c��Na+��+c��H+��+c��H2CO3������A����
B��������5mL 0.02mol/L HCl��Һ�к���������1��10-4mol��5mL 0.02mol/L Ba��OH��2��Һ�к���1��10-4mol���������ӣ�����ַ�Ӧ����Һ��ʣ��1��10-4mol���������ӣ������Һ���Ϊ10mL����Ӧ�����Һ������������Ũ��Ϊ��
=0.01mol/L������ҺpH=12����B��ȷ��
C����ͬ������pH=5�Ģ�NH4Cl��Һ ��CH3COOH��Һ ��ϡ���ᣬ�Ȼ����笠�����ˮ�⣬�ٽ���ˮ�ĵ��룬��Һ�е�������Ϊˮ����ģ���ϡ����ʹ�����Һ������ˮ�ĵ��룬��Һ�е�������������ˮ����ģ����ڶ���pH��ȣ���ˮ�ĵ���̶���ȣ�����������Һ����ˮ���������c��H+����СΪ���٣���=�ۣ���C����
D�������£���ϡNaOH��Һ��CH3COOH��Һ��ϣ����ܳ���pH��7����c��OH-����c��H+�������Dz������c��OH-����c��Na+����c��H+����c��CH3COO-������D����
��ѡB��
B��������5mL 0.02mol/L HCl��Һ�к���������1��10-4mol��5mL 0.02mol/L Ba��OH��2��Һ�к���1��10-4mol���������ӣ�����ַ�Ӧ����Һ��ʣ��1��10-4mol���������ӣ������Һ���Ϊ10mL����Ӧ�����Һ������������Ũ��Ϊ��
| 1��10-4mol |
| 0.01L |
C����ͬ������pH=5�Ģ�NH4Cl��Һ ��CH3COOH��Һ ��ϡ���ᣬ�Ȼ����笠�����ˮ�⣬�ٽ���ˮ�ĵ��룬��Һ�е�������Ϊˮ����ģ���ϡ����ʹ�����Һ������ˮ�ĵ��룬��Һ�е�������������ˮ����ģ����ڶ���pH��ȣ���ˮ�ĵ���̶���ȣ�����������Һ����ˮ���������c��H+����СΪ���٣���=�ۣ���C����
D�������£���ϡNaOH��Һ��CH3COOH��Һ��ϣ����ܳ���pH��7����c��OH-����c��H+�������Dz������c��OH-����c��Na+����c��H+����c��CH3COO-������D����
��ѡB��
���������⿼��������Ũ�ȴ�С�Ƚϡ���ҺpH�ļ��㡢����ˮ��ԭ����֪ʶ����ȷ���ǿ�����������ˮ��̶ȴ�С֮��Ĺ�ϵ�ǽⱾ��ؼ����ٽ���غ�˼����������Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �»ƸԱ����ܾ�ϵ�д�
�»ƸԱ����ܾ�ϵ�д�
�����Ŀ
��Al��ϡH2SO4�ķ�Ӧ�У���֪10sĩH2SO4��Ũ�ȼ�����0.6mol?L-1���������Ƿ�Ӧ��������Һ����ı仯����10s������Al2��SO4��3��ƽ����Ӧ����Ϊ��������
| A��0.02 mol?L-1?s-1 |
| B��0.04 mol?L-1?s-1 |
| C��0.06 mol?L-1?s-1 |
| D��0.18 mol?L-1?s-1 |
��ij��Һ����K+��H+��SO42-��NO3-������Һ�����ʵ��������Ϊ��������
| A��2�� | B��3�� | C��4�� | D��5�� |
��ˮ�м���������KHSO4���壨�¶Ȳ��䣩������Һ�ģ�������
| A��pHֵ���� |
| B��c��H+����c��OH-���ij˻����� |
| C��������ǿ |
| D��OH-����Ũ������ |
��60g�ɼ������ϩ��ɵĻ������ͨ��ʢ��������ˮ�������ʢ��ˮ������������������28g����ԭ������м������ϩ�����ʵ���֮��Ϊ��������
| A��1��2 | B��2��1 |
| C��3��2 | D��2��3 |
Ԫ�����ʳ������Ա仯�ľ��������ǣ�������
| A��Ԫ��ԭ�Ӱ뾶��С�������Ա仯 |
| B��Ԫ��ԭ�ӵ�ԭ�������������� |
| C��Ԫ�ص���������ϼ۳������Ա仯 |
| D��Ԫ��ԭ�����������Ų��������Ա仯 |
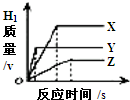 ����ͬ�������ͬ���������̵ֽ�ϡ���ᣬ�ֱ���뵽��������������С��ͬ��X��Y��Z���ֽϻ��ý����У�������ȫ��Ӧ��������H2�������뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ������˵������ȷ���ǣ�������
����ͬ�������ͬ���������̵ֽ�ϡ���ᣬ�ֱ���뵽��������������С��ͬ��X��Y��Z���ֽϻ��ý����У�������ȫ��Ӧ��������H2�������뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ������˵������ȷ���ǣ�������| A�����ֽ����Ļ��˳��ΪY��X��Z |
| B����������������˳��ΪX��Y��Z |
| C������ϡ���������˳��ΪZ��Y��X |
| D�����ԭ�������ɴ�С��˳��ΪX��Y��Z |
��NA��ʾ�����ӵ�������ֵ������������ȷ���ǣ�������
| A�������£�18g H2O�к�H2��ĿΪNA |
| B����״���£�22.4L H2O�к��л�ѧ������ĿΪ2NA |
| C����0.5mol SO2ͨ������ˮ�У���ַ�Ӧ��ɵõ�H2 SO3������Ϊ0.5NA |
| D�����³�ѹ�£�Na2O2������H2O��Ӧ��������0.2mol O2��ת�Ƶ��ӵ���ĿΪ0.4NA |