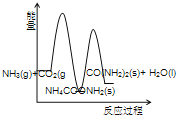
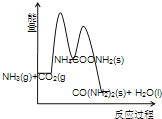
��Ŀ����
����Ŀ��ij�����A������KAl(SO4)2��Al2O3 ��Fe2O3����һ�������¿�ʵ����ͼ��ʾ������֮��ı仯��
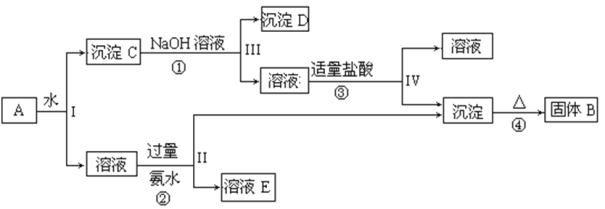
�ݴ˻ش��������⣺
(1)I��II��III��IV�IJ��ж�����Һ�ͳ����ķ����ȡ�ķ�����____________��
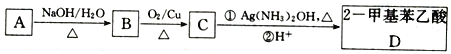
(2)����������ͼ��Ӧ��ϵ��д������B��C��D�������ʵĻ�ѧʽ����B _______�� ����C ________ �� ����D ________��
(3)д���١��ڡ��۵����ӷ���ʽ�ܵ͢Ļ�ѧ����ʽ��
��__________________________________________
��_________________________________________
��_________________________________________
�� ________________________________________
���𰸡����� Al2O3 Al2O3��Fe2O3 Fe2O3 Al2O3+2OH-=2AlO2-+H2O Al3++3NH3H2O�TAl(OH)3��+3NH4+ AlO2-+ H++H2O= Al(OH)3�� 2Al(OH)3 ![]() Al2O3+3H2O
Al2O3+3H2O
��������
�����A�к���KAl(SO4)2��Al2O3��Fe2O3����ˮ���˷���ɵ���ҺKAl(SO4)2��Al2O3��Fe2O3�����ij���C������C�м���NaOH��Һ��NaOH��Al2O3��Ӧ����NaAlO2�����˺�õ�NaAlO2��Һ������DΪFe2O3�� NaAlO2��Һ�м����������ᣬ��Ӧ����Al(OH)3��NaCl��Al(OH)3���ȷֽ����ɹ���BΪAl2O3��KAl(SO4)2��Һ�м��������ˮ���õ�����������������ҺE��E�к���K2SO4��(NH4)2SO4��NH3H2O��������ʵ����ʷ������
(1)I��II��III��IV�IJ��ж�����Һ�ͳ����ķ����ȡ�ķ����ǹ��ˣ��ʴ�Ϊ�����ˣ�
(2)��������ķ�����֪������BΪAl2O3������CΪAl2O3��Fe2O3�Ļ�������DΪFe2O3���ʴ�Ϊ��Al2O3��Al2O3��Fe2O3��Fe2O3��
(3)��Ӧ�ٵ����ӷ���ʽΪAl2O3+2OH-=2AlO2-+H2O����Ӧ�ڵ����ӷ���ʽΪAl3++3NH3H2O�TAl(OH)3��+3NH4+����Ӧ�۵����ӷ���ʽΪAlO2-+ H++H2O= Al(OH)3������Ӧ�ܵĻ�ѧ����ʽΪ2Al(OH)3 ![]() Al2O3+3H2O���ʴ�Ϊ��Al2O3+2OH-=2AlO2-+H2O��Al3++3NH3H2O�TAl(OH)3��+3NH4+��AlO2-+ H++H2O= Al(OH)3����2Al(OH)3
Al2O3+3H2O���ʴ�Ϊ��Al2O3+2OH-=2AlO2-+H2O��Al3++3NH3H2O�TAl(OH)3��+3NH4+��AlO2-+ H++H2O= Al(OH)3����2Al(OH)3 ![]() Al2O3+3H2O��
Al2O3+3H2O��