��Ŀ����
10����1�����и����е����ֹ�̬�����ۻ�����������ʱ���˷������������������ͬ�����͵���C��ѡ��A��B��C��D����A ��͵⻯�� B ���ʯ���ؾ�ʯ
C �������Ӳ֬������� D �ɱ��Ͷ�������
��2���������V���������ͱ��������Һ���������2V������ڡ����ڡ�С�ڡ���ȷ����
��3��������N2H4���백���ƣ�д�������Ľṹʽ
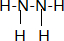 ����N���ӻ������sp3������Ҳ�м��ԣ���������ڰ� ���ǿ�ڡ����ڣ����������ȶ������ڰ����ǿ�ڡ����ڣ�
����N���ӻ������sp3������Ҳ�м��ԣ���������ڰ� ���ǿ�ڡ����ڣ����������ȶ������ڰ����ǿ�ڡ����ڣ���4���Ȼ��ƾ����У�������֮����̾���Ϊa cm���ܶ�Ϊb g/cm3����Ħ������ΪM g/mol����NA=$\frac{\sqrt{2}M}{2{a}^{3}b}$/mol ����M��a��b��ʾ��
��5��PF3��NF3 ��NH3�ṹ���ƣ����Ǵ�С˳��ΪNH3��NF3��PF3��
���� ��1����̬��������ʱ�����Ӿ���˷����Ӽ���������ԭ�Ӿ���˷���ѧ�������Ӿ���˷����Ӽ�����������˷���������
��2����������������֮������������ȱ����Ӽ���������ʹ�����Ӽ����������Ҫ����
��3�����������е�һ��Hԭ�ӱ�����ȡ��Ϊ�������ݴ���д�����ṹʽ���÷�����Nԭ�Ӽ۲���ӶԸ�����4�Һ���һ���µ��Ӷԣ����ݼ۲���ӶԻ��������ж�Nԭ���ӻ���ʽ��
N2H4�൱��NH3��һ��Hԭ�ӻ�����-NH2����ȻNԭ�ӵ�����������Զǿ��Hԭ�ӣ����N2H4�е�Nԭ���ϵ������ܶ�С��NH3�����ѽ��H+����������
��������ΪH2N-N�����Ĺ��õ��ӶԱ�H2N-H�Ĺ��õ��ӶԸ�Զ����ߵ�N��
�����У�����ԽС�÷���Խ���ȶ���
��4���Ȼ��ƾ����У�������֮����̾���Ϊa cm�����ⳤΪ$\sqrt{2}$acm���������=��$\sqrt{2}$acm��
3���þ����������Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4�������Ӹ���=12��$\frac{1}{4}$+1=4������٤������=$\frac{4M}{��V}$��
��5��PF3��NF3 ��NH3�ṹ���Ƶ��������νṹ����λԭ����ͬʱ������ԭ�ӵĵ縺��Խ����Խ��
��� �⣺��1����̬��������ʱ�����Ӿ���˷����Ӽ���������ԭ�Ӿ���˷���ѧ�������Ӿ���˷����Ӽ�����������˷���������
A�����Ƿ��Ӿ��塢�⻯�������Ӿ��壬����ʱ��ǰ���ƻ����Ӽ��������������ƻ����Ӽ������Կ˷�������������ͬ���ʲ�ѡ��
B�����ʯ��ԭ�Ӿ��塢�ؾ�ʯ�����Ӿ��壬����ʱǰ���ƻ���ѧ���������ƻ����Ӽ������Կ˷�������������ͬ���ʲ�ѡ��
C���������Ӳ֬����������Ƿ��Ӿ��壬����ʱ�����ƻ����Ӽ���������������ͬ����ѡ��
D���ɱ��Ƿ��Ӿ��塢����������ԭ�Ӿ��壬����ʱǰ���ƻ����Ӽ��������������ƻ���ѧ�������Կ˷�������������ͬ���ʲ�ѡ��
��ѡC��
��2�����ͱ������Ϻ�������������֮������������ȱ����Ӽ���������ʹ�����Ӽ����������Ҫ�������Ա����������Ӽ��ӴӶ�ʹ�������������Һ���֮�ͣ��ʴ�Ϊ�����ڣ�
��3�����������е�һ��Hԭ�ӱ�����ȡ��Ϊ�����������ṹʽΪ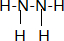 ���÷�����Nԭ�Ӽ۲���ӶԸ�����4�Һ���һ���µ��Ӷԣ����ݼ۲���ӶԻ������۵�Nԭ���ӻ���ʽΪsp3��
���÷�����Nԭ�Ӽ۲���ӶԸ�����4�Һ���һ���µ��Ӷԣ����ݼ۲���ӶԻ������۵�Nԭ���ӻ���ʽΪsp3��
N2H4�൱��NH3��һ��Hԭ�ӻ�����-NH2����ȻNԭ�ӵ�����������Զǿ��Hԭ�ӣ����N2H4�е�Nԭ���ϵ������ܶ�С��NH3�����ѽ��H+����������
��������ΪH2N-N�����Ĺ��õ��ӶԱ�H2N-H�Ĺ��õ��ӶԸ�Զ����ߵ�N��
�ʴ�Ϊ��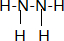 ��sp3�����ڣ����ڣ�
��sp3�����ڣ����ڣ�
��4���Ȼ��ƾ����У�������֮����̾���Ϊa cm�����ⳤΪ$\sqrt{2}$acm���������=��$\sqrt{2}$acm��3���þ����������Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4�������Ӹ���=12��$\frac{1}{4}$+1=4������٤������=$\frac{4M}{��V}$=$\frac{4M}{��\sqrt{2}a��^{3}b}$/mol=$\frac{\sqrt{2}M}{2{a}^{3}b}$/mol��
�ʴ�Ϊ��$\frac{\sqrt{2}M}{2{a}^{3}b}$/mol��
��5�������У�����ԽС�÷���Խ���ȶ���NF3��PF3����λԭ�Ӷ���F��N�ĵ縺�Դ���P������NF3�ļ��Ǵ���PF3��NF3��NH3������ԭ����ͬ��F�ĵ縺�Դ���H������NF3�ļ���С��NH3����˼���NH3��NF3��PF3���ʴ�Ϊ��NH3��NF3��PF3��
���� ���⿼�����ʽṹ�����ʣ����ؿ���ѧ���ռ����������������������漰�������������������㡢���ǵ�֪ʶ�㣬�ѵ��Ǿ������㣬�״����ǣ�2���⣬��Ŀ�Ѷ��еȣ�

| A�� | CԪ�صĵ�����BԪ�صĵ�����ȼ�գ�����1mol��������ˮ��Ӧת�Ƶ�����ΪNA | |
| B�� | ������Ԫ���γɵĻ�����ֻ��һ�־���Ư���� | |
| C�� | B��C��D����Ԫ���γɵĻ������ˮ��Һ�ö��Ե缫���ʱ����ҺpHһ������ | |
| D�� | A��B�γɻ�����ķе����A��C�γɻ�����ķе㣬��Ϊǰ�߷��Ӽ��γ������ |
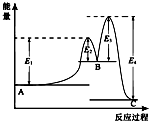 ij��Ӧ��������ӦA?B?C���ɣ����ķ�Ӧ����������ͼ��ʾ��E1��E2��E3��E4��ʾ��ܣ��������й�������ȷ���ǣ�������
ij��Ӧ��������ӦA?B?C���ɣ����ķ�Ӧ����������ͼ��ʾ��E1��E2��E3��E4��ʾ��ܣ��������й�������ȷ���ǣ�������| A�� | ������Ӧ��Ϊ���ȷ�Ӧ | B�� | ������Ӧ�С�H=E1+E3-E2-E4 | ||
| C�� | ���������ı䷴Ӧ���ʱ� | D�� | ���ֻ�������B���ȶ� |
| ѡ�� | �������ʵ | ������˵�� |
| A | ƻ�����ڿ����о��ñ�ƺ�ֽ�ž��ñ�� | ���߱�Ƶ�ԭ������ |
| B | �����Ȼ�̼�����·��ĸ�ϴ | Ŀ���ǽ�Լ��ˮ |
| C | ������Ʒ��ͭ��Ʒ�ڿ����б���ʴ | �����ܷ���������ʴ���ܷ������ⸯʴ |
| D | �γɻƺ��뺣��ɳ�ޡ���±ˮ�㶹�� | �������˽���۳������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | NH4+��CH3COO-��Ca2+��Cl- | B�� | Ca2+��Ag+��SO42-��NO3- | ||
| C�� | Cu2+��H+��S2-��NO3- | D�� | C6H5O-��Fe3+��H+��Cl- |
| ѡ�� | ʵ����������� | ʵ��Ŀ�Ļ���� |
| A | �������ữ��H2O2��Һ����Fe��NO3��2��Һ�У���Һ���ɫ | ��֤�������ԣ�H2O2��Fe3+ǿ |
| B | ��0.1mol/L��NaHCO3��Һ�У���2�η�̪��dz��ɫ���ȣ���Һ��ɫ���� | ��֤����ˮ�ⷴӦ�����ȷ�Ӧ |
| C | ��һ������NaNO3��KCl�Ļ��Һ���Ȳ�Ũ�����о������������ȹ��� | �õ�NaCl���� |
| D | �����ᱵ����������ᣬ�ж��������������� | ���������ǿ�������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | Na2CO3��NaOH��������ˮ�ĵ��� | |
| B�� | 0.1 mol•L-1Na2CO3��Һ��ˮϡ�ͣ�CO32-��ˮ��̶�������ҺpH��С | |
| C�� | ����к͵ζ�ʵ���У���ƿ���ô���Һ��ϴ2��3�κ��ټ������Һ | |
| D�� | �����£�pH=3�����ᡢ����ֱ���ˮϡ��m����n����pH��ͬ����m��n |
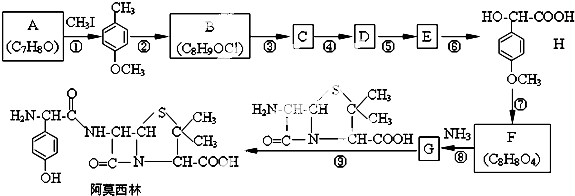
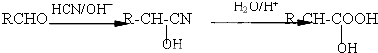
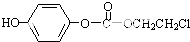 ����������NaOHˮ��Һ�м��ȷ�Ӧʱ�Ļ�ѧ����ʽΪ
����������NaOHˮ��Һ�м��ȷ�Ӧʱ�Ļ�ѧ����ʽΪ +5NaOH$��_{��}^{H_{2}O}$
+5NaOH$��_{��}^{H_{2}O}$ +HOCH2CH2OH+Na2CO3+NaCl+2H2O��
+HOCH2CH2OH+Na2CO3+NaCl+2H2O�� ��
��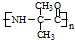 �ĺϳ�·������ͼ�����Լ����ã���
�ĺϳ�·������ͼ�����Լ����ã���