��Ŀ����
2�����ڸ�����Һ����Ũ�ȵĹ�ϵ��ȷ���ǣ�������| A�� | ��һ��������ͨ��0.1 mol/L NH4Cl��Һ�п����У�c��Cl-����c��NH4+����c��OH-����c��H+�� | |
| B�� | �����ʵ�����NaClO��NaHCO3�Ļ����Һ��һ���У�c��HClO��+c��ClO-��=c�� HCO3-��+c�� H2CO3��+c��CO32-�� | |
| C�� | ��CH3COONa��Һ�еμ�ϡ��������Һ������ʱ�У�c��Cl-����c�� Na+����c�� CH3COOH�� | |
| D�� | ��Ũ�ȵ������CH3COOH��Һ��CH3COONa��Һ��Ͼ��Ⱥ�c��CH3COO-��+c��CH3COOH��=c��Na+�� |
���� A�����ݵ���غ����������ɵ���Ũ�ȵ��ڸ���ɵ���Ũ�ȣ�
B�����������غ��������Һ��CԪ�ص������ʵ�������ClԪ�ص������ʵ�����
C����Һ�����ԣ���c��H+��=c��OH-�������ݵ���غ������
D�����������غ������
��� �⣺A����һ��������ͨ��0.1 mol/L NH4Cl��Һ�У������غ�Ϊ��c��Cl-��+c��OH-��=c��NH4+��+c��H+������c��Cl-����c��NH4+������c��H+����c��OH-������A����
B�������ʵ�����NaClO��NaHCO3�Ļ����Һ�У�CԪ�ص������ʵ�������ClԪ�ص������ʵ�����������Һ��һ���У�c��HClO��+c��ClO-��=c�� HCO3-��+c�� H2CO3��+c��CO32-������B��ȷ��
C����CH3COONa��Һ�еμ�ϡ��������Һ�����ԣ���c��H+��=c��OH-�����ɵ���غ��֪��c��Cl-��+c��CH3COO-��+c��OH-��=c��Na+��+c��H+�������ԣ� Na+����c��Cl-������C����
D����Ũ�ȵ������CH3COOH��Һ��CH3COONa��Һ��ϣ���Һ��c��CH3COO-��+c��CH3COOH��=2c��Na+������D����
��ѡB��
���� ���⿼������Һ������Ũ�ȴ�С�Ƚϣ�ע����յ���غ�������غ��Ӧ�ã���Ŀ�ѶȲ������ڻ���֪ʶ�Ŀ��飮

| A�� | �����£�0.1 mol•L-1��������Һ�к���Na+��CH3COO-������Ϊ0.2NA | |
| B�� | �����£���0.1 mol��Ƭ��������Ũ�����з�Ӧ��ת�Ƶ��ӵ���ĿΪ0.3NA | |
| C�� | 2 g NO2��44 g N2O4�Ļ������������ԭ�ӵ�����Ϊ3NA | |
| D�� | 0.1 mol����ȩ�к���˫������ĿΪ0.3NA |
| A�� | ����Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳���ǣ�M��W��Z��Y��X | |
| B�� | ������X2W2��YW2��ZW2�ȶ����м��Թ��ۼ��ͷǼ��Թ��ۼ� | |
| C�� | ��M������������ʯī�缫���������NaHCO3��Һ�����һ��ʱ��������������ְ�ɫ���� | |
| D�� | X��Z��Ԫ�����γ�ԭ�Ӹ����ȣ�X��Z��Ϊ3��1��2��1�Ļ����� |
 ��һ�ܱ������м���A��B�������ʵ����ʵ���Ũ�����ŷ�Ӧ�Ľ��У���ͼ��ʾ������˵������ȷ���ǣ�������
��һ�ܱ������м���A��B�������ʵ����ʵ���Ũ�����ŷ�Ӧ�Ľ��У���ͼ��ʾ������˵������ȷ���ǣ�������| A�� | �÷�Ӧ�Ļ�ѧ����ʽΪ5A+4B?4C | |
| B�� | 2minʱ���÷�Ӧ�ﵽƽ�⣬��ʱA��B��C��Ũ�ȱ�Ϊ5��4��4 | |
| C�� | ��B��Ũ�ȱ仯��ʾ2min�ڵ�����Ϊ2mol/��L•min�� | |
| D�� | 2minǰ������Ӧ������С���淴Ӧ���������� |
| A�� | 9�� | B�� | 16�� | C�� | 19�� | D�� | 22�� |
| A�� | 100mL 3.0mol/L��������5.6g��м��ȫ��Ӧת�Ƶĵ�����Ϊ0.3NA | |
| B�� | ��2mol H2SO4��Ũ��������������ͭ��ȫ��Ӧ������SO2����ĿΪNA | |
| C�� | 1L 0.1mol/L Na2CO3��Һ�У�������������Ϊ0.3NA | |
| D�� | 16.0g�Ĺ��������������к��еĵ�����Ϊ9NA |
| A�� | ������������Ӧ����ϵ���������� | |
| B�� | ��Ҫ���Ȳ��ܷ����ķ�Ӧһ�������ȷ�Ӧ | |
| C�� | ������������������ڷ�Ӧ�������������Ӧ������������ | |
| D�� | ��ѧ��Ӧ��ʵ���Ǿɻ�ѧ���Ķ��Ѻ��»�ѧ�����γ� |
| A�� | ��ɫ��Һ��Cu2+��K+��MnO4-��SO42- | |
| B�� | ���ܽ�Al2O3����Һ��Na+��Ca2+��HCO3-��NO3- | |
| C�� | ������c��OH-��=1��10-13mol•L-1����Һ��NH4+��Al3+��SO42-��Cl- | |
| D�� | ��1.2 mol•L-1 NO3-����Һ��H+��Fe2+��Mg2+��Br- |

 ����
���� ����ԭ�ӽ���ˮ�⣮
����ԭ�ӽ���ˮ�⣮ ��
�� ��
�� ��
��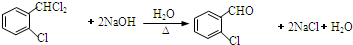 ��
�� ��
�� ��
�� ��д������ϩ���״�Ϊ�л�ԭ���Ʊ�������
��д������ϩ���״�Ϊ�л�ԭ���Ʊ������� �ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�CH3CH2OH$��_{170��}^{ŨH_{2}SO_{4}}$CH2=CH2$\stackrel{Br_{2}}{��}$
�ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�CH3CH2OH$��_{170��}^{ŨH_{2}SO_{4}}$CH2=CH2$\stackrel{Br_{2}}{��}$