��Ŀ����
18��ͨ����ʳ���Ϳɻ��ij�����л�������X������Է�������Ϊ46������̼����������Ϊ52.2%�������������Ϊ13.0%����1��X�ĵ���ʽ��
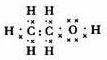 ��
����2��X������Ʒ�Ӧ�ų���������Ӧ�Ļ�ѧ����ʽ��2C2H5OH+2Na��2C2H5ONa+H2�����л����ýṹ��ʽ�����
��3��X������е�������ͭ�������·�Ӧ����Y��Y�Ľṹ��ʽ��CH3CHO��Y�ܷ���������Ӧ��Y��������Һ�ķ�Ӧ�Ļ�ѧ����ʽ�ǣ�CH3CHO+2Ag��NH3��2OH$\stackrel{ˮԡ����}{��}$CH3COONH4+2Ag��+3NH3+H2O��
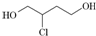
���� ��1��������֪������������Ϊ��1-52.2%-13.0%=34.8%����̼���⡢��ԭ�ӵĸ����ֱ�Ϊ��N��C��=$\frac{46��52.2%}{12}$��2��N��H��=$\frac{46��13.0%}{1}$��6��N��O��=$\frac{46��34.8%}{16}$��1����X�ķ���ʽΪC2H6O����XΪ�Ҵ����Ҵ�Ϊ���ۻ���������к��й������ǻ����ݴ�д���Ҵ��ĵ���ʽ��
��2���Ҵ����������ǻ����ʺ��Ʒ�Ӧ�����������ݴ��Ҵ����Ʒ�Ӧ�Ļ�ѧ����ʽ��
��3���Ҵ�������������ȩ����ȩ�ܹ���������Һ����������Ӧ��
��� �⣺��1��������������Ϊ��1-52.2%-13.0%=34.8%����̼���⡢��ԭ�ӵĸ����ֱ�Ϊ��N��C��=$\frac{46��52.2%}{12}$��2��N��H��=$\frac{46��13.0%}{1}$��6��N��O��=$\frac{46��34.8%}{16}$��1����X�ķ���ʽΪC2H6O�����л���Ϊ�Ҵ����Ҵ�Ϊ���ۻ������ṹʽΪ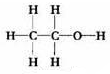 ��ÿһ�����ߴ���һ�Ե��ӣ��������ʽΪ��
��ÿһ�����ߴ���һ�Ե��ӣ��������ʽΪ��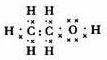 ��
��
�ʴ�Ϊ��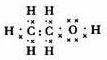 ��
��
��2���Ҵ����Ʒ�Ӧ������������Ӧ����ʽΪ��2C2H5OH+2Na��2C2H5ONa+H2�����ʴ�Ϊ��2C2H5OH+2Na��2C2H5ONa+H2����
��3���Ҵ��к����ǻ����ܹ���������������ȩ��CH3CH������ȩ�к���ȩ�����ܹ�����������Ӧ����Ӧ����ʽΪ��CH3CHO+2Ag��NH3��2OH $\stackrel{ˮԡ����}{��}$ CH3COONH4+2Ag��+3NH3+H2O��
�ʴ�Ϊ��CH3CHO��CH3CHO+2Ag��NH3��2OH $\stackrel{ˮԡ����}{��}$ CH3COONH4+2Ag��+3NH3+H2O��
���� ���⿼�����л������ʽ���ṹ��ʽ��ȷ������Ŀ�Ѷ��еȣ�ע�����ճ����л���ṹ�����ʣ���ȷ�����غ㶨����ȷ���л������ʽ�е�Ӧ�÷���������������ѧ���ķ�����������ѧ����������

 ��һ���м�������˵����ȷ���ǣ�������
��һ���м�������˵����ȷ���ǣ�������| A�� | 1��3����ϩ����ʽΪC4H8 | |
| B�� | 1��3����ϩת��Ϊ ʱ������Cl2����1��4�ӳ���ˮ��õ� ʱ������Cl2����1��4�ӳ���ˮ��õ� | |
| C�� |  ��NaOH����Һ�л�Ũ�������ʱ���ȶ��ܷ�����ȥ��Ӧ ��NaOH����Һ�л�Ũ�������ʱ���ȶ��ܷ�����ȥ��Ӧ | |
| D�� |  ��������X��X�ܷ���������Ӧ����X�Ľṹֻ������ ��������X��X�ܷ���������Ӧ����X�Ľṹֻ������ |
| A�� | ��������X2ȫ��ת��Ϊ����ͬ��������X3����ôX3�ķ�������3�� | |
| B�� | ������Ϊ16��һ�ֺ����ڴ������е�ԭ�Ӱٷ���Ϊ80% | |
| C�� | ֻҪ������ͬλ����ɲ��䣬Xԭ�����κ������·���������ϣ����õ�����X2��������֮������15��4��1 | |
| D�� | ��������X2��ƽ����������32.6 |
| A�� | ���ӵ��ȶ����뻯ѧ����ǿ���� | |
| B�� | �μӷ�Ӧ�����������������������ڷ�Ӧ����ˮ�������� | |
| C�� | �Ͽ��μӷ�Ӧ�������������еĻ�ѧ�����յ�������������ˮʱ�γɻ�ѧ���ų������� | |
| D�� | �÷�Ӧ��Ҫ��ȼ�����������ȷ�Ӧ |
| A�� | ����91.5 kJ | B�� | ����183 kJ | C�� | ����183 kJ | D�� | ����91.5 kJ |
| A�� | ���³�ѹ�£�7.0g��ϩ���ϩ�Ļ�����к�����ԭ�ӵ���ĿΪNA | |
| B�� | ���³�ѹ�£�22.4 L CH4�к��е�C-H����Ϊ4NA | |
| C�� | ��0.2 mol H2SO4��Ũ����������Cu��Ӧ������SO2�ķ�����Ϊ0.1NA | |
| D�� | lmol FeCl2������������Ӧʱת�Ƶĵ�����Ϊ2NA |
| A�� | 11��17 | B�� | 19��9 | C�� | 13��17 | D�� | 20��8 |
 ��
�� ��CH3CH3��
��CH3CH3��
 +3HNO3 $��_{��}^{Ũ����}$
+3HNO3 $��_{��}^{Ũ����}$ +3H2O��
+3H2O��