��Ŀ����
7��д�����з�Ӧ�Ļ�ѧ����ʽ��1����������ȡһ������C2H6+Cl2$\stackrel{����}{��}$C2H5Cl+HCl
��2����ϩ��ˮ��ӦCH2=CH2+H2O$\stackrel{����}{��}$CH3-CH2-OH
��3����������һ�������·�ӦC6H6+Br2$\stackrel{Fe}{��}$C6H5Br+HBr
��4���ɼױ���ȡTNT
 +3HNO3 $��_{��}^{Ũ����}$
+3HNO3 $��_{��}^{Ũ����}$ +3H2O��
+3H2O��
���� ��1���������巢��ȡ����Ӧ���������飻
��2����ϩ��ˮ�ӳ������Ҵ���
��3������Һ�����������������£�����ȡ����Ӧ�����屽���廯�⣻
��4���ױ���Ũ������Ũ������������¼�������2��4��6-�������ױ���
��� �⣺��1����������������ȡ����Ӧ�����������HCl���÷�ӦΪC2H6+Cl2$\stackrel{����}{��}$C2H5Cl+HCl��
�ʴ�Ϊ��C2H6+Cl2$\stackrel{����}{��}$C2H5Cl+HCl��
��2����ϩ��ˮ�ӳ������Ҵ�������ʽ��CH2=CH2+H2O$\stackrel{����}{��}$CH3-CH2-OH��
�ʴ�Ϊ��CH2=CH2+H2O$\stackrel{����}{��}$CH3-CH2-OH��
��3�����������������������·���ȡ����Ӧ������ʽ��C6H6+Br2$\stackrel{Fe}{��}$C6H5Br+HBr��
�ʴ�Ϊ��C6H6+Br2$\stackrel{Fe}{��}$C6H5Br+HBr��
��4���ױ�����ȡ����Ӧ����Ũ���ᡢŨ����Ļ���Ṳ��ʱ����������Ӧ�����������ױ�������ʽΪ +3HNO3 $��_{��}^{Ũ����}$
+3HNO3 $��_{��}^{Ũ����}$ +3H2O��
+3H2O��
�ʴ�Ϊ�� +3HNO3 $��_{��}^{Ũ����}$
+3HNO3 $��_{��}^{Ũ����}$ +3H2O��
+3H2O��
���� ���⿼���л���ѧ��Ӧ����ʽ����д��Ϊ��Ƶ���㣬�������ʵ����ʼ������Ļ�ѧ��ӦΪ���Ĺؼ���ע���л���Ӧ����������Ŀ�ѶȲ���

| A�� | A��NH3��g��+$\frac{5}{4}$O2��g���TNO��g��+$\frac{6}{4}$H2O��g������H=-akJ•mol-1 | |
| B�� | C6H12O6��s��+6O2��g���T6CO2��g��+6H2O��l������H=-bkJ•mol-1 | |
| C�� | 2CO��g��+O2��g���T2CO2��g������H=-ckJ•mol-1 | |
| D�� | CH3CH2OH��l��+$\frac{1}{2}$O2��g���TCH3CHO��l��+H2O��l������H=-dkJ•mol-1 |
| A�� | ����ȫȼ�գ�1 mol��ͪ�� ���ȴ�ͪ�� ���ȴ�ͪ�� ��������2 mol O2 ��������2 mol O2 | |
| B�� | �������е�һ��̼ԭ�ӱ�һ����ԭ�Ӵ��棬���·��ӵ�ʽ��Ϊ79 | |
| C�� | ���ǡ���ѿ�Ǻ����ǵķ���ʽ��ΪC12H22O11�����ܷ���������Ӧ | |
| D�� | ֻ����ˮһ���Լ����ܼ���ױ�����ϩ���Ҵ������Ȼ�̼����Һ�� |
| A�� | 1 mol�ױ�����6NA��C-H�� | |
| B�� | 0.1 molCH2=CH-COOH�к���˫������ĿΪ0.1NA | |
| C�� | ��״���£�11.2 L���к��з��ӵ���ĿΪ0.5NA | |
| D�� | ��ϩ����ϩ��ɵ�56 g�����������ԭ�ӵĸ���Ϊ8NA |
| A�� | ��ȩ | B�� | ��ȩ | C�� | ���� | D�� | ���� |
 �����ŷŵ�β��Ϊ������ȾԴ֮һ��Ŀǰ�����������»�ѧԭ�����β����
�����ŷŵ�β��Ϊ������ȾԴ֮һ��Ŀǰ�����������»�ѧԭ�����β����2NO+2CO$\stackrel{����}{?}$2CO2+N2��
��1����֪2molNO��ȫת���ų�akJ����������2molNO ��2molCO���������з�Ӧ���ų�0.8akJ�����������N2�����ʵ���Ϊ0.8mol��
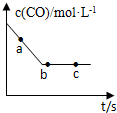
��2��һ�������£����ݻ��̶��������н���������Ӧ��COŨ����ʱ���ϵ��ͼ��ʾ��
����Ӧ���ʦԣ�a�����ԣ�b�����ԣ�c���Ĵ�С��ϵ�Ǧԣ�a�����ԣ�b��=�ԣ�c��
�����п�˵����Ӧ����ͼ��c�����BC
A��NO��CO��CO2��N2��Ũ�ȱ�ֵ����2��2��2��1����
B��CO��Ũ�Ȳ��ٸı�
C����Ӧ����2molNO��ͬʱ����1molN2
��3��Ϊ�о������߸�ת�����̷�Ӧ���ʣ�ij���������������ʵ��̽����
�����ϲ��ġ�
A����ͬ�Ĵ�����ͬһ��Ӧ�Ĵ�Ч�ʲ�ͬ��
B��ʹ�õ�������ͬ�Ĵ���ʱ�������ıȱ�����Դ�Ч����Ӱ�죮
��ʵ����ơ�������Ϊ̽��ijЩ�������������β��ת����Ӧ���ʵ�Ӱ����ɣ���������¶Ա�ʵ�飮
| ʵ���� | ʵ��Ŀ�� | T/�� | NO��ʼŨ�� mol/L | CO��ʼŨ�� mol/L | ͬ�ִ����ıȱ���� m2/g | ��ƽ��ʱ���õ�ʱ��min |
| �� | ����ʵ�� | 280 | 6.50��10-3 | 4.00��10-3 | 80 | t |
| �� | 280 | 6.50��10-3 | 4.00��10-3 | 120 | 0.5t | |
| �� | ̽���¶ȶԷ�Ӧ���ʵ�Ӱ�� | 360 | 6.50��10-3 | 4.00��10-3 | 80 | 0.2t |
�����ۡ�
���ɱ������ݿ�֪�������¶ȣ���Ӧ���ʽ��������������С��������Ӱ�족����
��֪���ٽ���Һ���е���������Ҫ��H+��Co2+��Fe2+��Mn2+��Al3+�ȣ�
�ڲ���������������������ʽ����ʱ��Һ��pH������
| ������ | Fe��OH��3 | Fe��OH��2 | Co��OH��2 | Al��OH��3 | Mn��OH��2 |
| ��ʼ���� | 2.7 | 7.6 | 7.6 | 4.0 | 7.7 |
| ��ȫ���� | 3.7 | 9.6 | 9.2 | 5.2 | 9.8 |
��2��д������Һ����NaClO3������Ӧ����Ҫ���ӷ���ʽClO3-+6Fe2++6H+=Cl-+6Fe3++3H2O
��3����Na2CO3��pH��5.2���ó���ΪFe��OH��3��Al��OH��3��
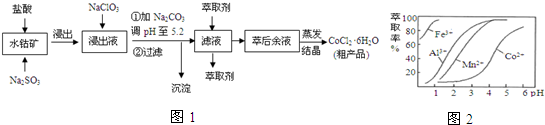
��4����ȡ���Խ������ӵ���ȡ����pH�Ĺ�ϵ��ͼ2����ȡ���������dz�ȥ��Һ�е�Mn2+����ʹ�õĽ�����pH��Χ��B��
A��2.0��2.5 B��3.0��3.5 C��4.0��4.5
��5��Ϊ�ⶨ�ֲ�Ʒ��CoCl2•6H2O��������ȡһ���� ���Ĵֲ�Ʒ����ˮ����������AgNO3��Һ�����ˡ�ϴ�ӣ���������ɺ����������ͨ�����㷢�ֲִ�Ʒ��CoCl2•6H2O��������������100%����ԭ������Ǵֲ�Ʒ���п������Ȼ������ʧȥ�˲��ֽᾧˮ������һ�����ɣ�
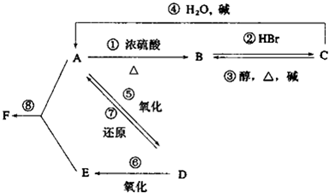 ij�л���A����C��H��O����Ԫ����ɣ���һ�������£���A����ת��Ϊ�л���B��C��D��E��C�ֿ���ת��ΪB��A�����ǵ�ת����ϵ��ͼ��
ij�л���A����C��H��O����Ԫ����ɣ���һ�������£���A����ת��Ϊ�л���B��C��D��E��C�ֿ���ת��ΪB��A�����ǵ�ת����ϵ��ͼ��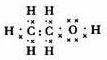 ��
��