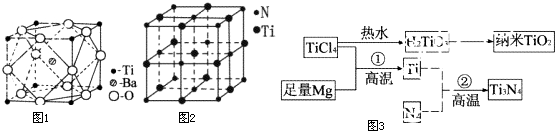
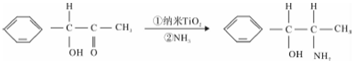
��Ŀ����
10���л���A��C6H8O4��ΪʳƷ��װֽ�ij��÷�������A������ˮ������ʹ���CCl4��Һ��ɫ��A������������ˮ�ⷴӦ���õ�B��C4H4O4����C��ͨ��״����BΪ��ɫ���壬��������������Һ������Ӧ��C��һ��ͬϵ��������㷺ʹ�õ����ϳɷ֣�
��1��A���Է����ķ�Ӧ�����Т٢ۢܣ�ѡ����ţ�
�ټӳɷ�Ӧ ��������Ӧ �ۼӾ۷�Ӧ ��������Ӧ
��2��B�������������ŵ�����̼̼˫�����Ȼ�
��3��B������û��֧������B�Ľṹ��ʽHOOCCH=CHCOOH����B������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽCH2=C��COOH��2
��4����B��ȡA�Ļ�ѧ��Ӧ����ʽHOOCCH=CHCOOH+2CH3OH$��_{��}^{Ũ����}$CH3OOCCH=CHCOOCH3+2H2O���÷�Ӧ����������Ӧ
��5�����Ŷ����ᣨC4H7NO4����������嵰���ʵİ�����֮һ�����������Ӧ���̣��ƶ����Ŷ�����Ľṹ��ʽHOOCCH2CH��NH2��COOH
��6�����������Ŷ�����һ�������¿�����������һ����Ԫ���Ľṹ�����ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ2 HOOCCH2CH��NH2��COOH$\stackrel{һ��������}{��}$
 +2H2O��
+2H2O��
���� ��A��������������ˮ���B��C����֪AΪ������ͨ��״����BΪ��ɫ���壬��������������Һ������Ӧ����BΪ���ᣬC���ڴ�������B�ķ���ʽΪC4H4O4����֪ÿ����B�к�2��-COOH�����A������ˮ������ʹ���CCl4��Һ��ɫ��˵��A�����к���̼̼�����ͼ�����B�г����Ȼ��⣬����C=C������ϣ�3����B����û��֧������BΪHOOCCH=CHCOOH��C��һ��ͬϵ��������㷺ʹ�õ����ϳɷ֣���CΪCH3OH����AΪCH3OOCCH=CHCOOCH3��B��HCl�����ӳɷ�Ӧ����CΪHOOCCH2CHClCOOH�������Ŷ�����Ľṹ��ʽ��HOOCCH2CH��NH2��COOH���ݴ˽��
��� �⣺��A��������������ˮ���B��C����֪AΪ������ͨ��״����BΪ��ɫ���壬��������������Һ������Ӧ����BΪ���ᣬC���ڴ�������B�ķ���ʽΪC4H4O4����֪ÿ����B�к�2��-COOH�����A������ˮ������ʹ���CCl4��Һ��ɫ��˵��A�����к���̼̼�����ͼ�����B�г����Ȼ��⣬����C=C������ϣ�3����B����û��֧������BΪHOOCCH=CHCOOH��C��һ��ͬϵ��������㷺ʹ�õ����ϳɷ֣���CΪCH3OH����AΪCH3OOCCH=CHCOOCH3��B��HCl�����ӳɷ�Ӧ����CΪHOOCCH2CHClCOOH�������Ŷ�����Ľṹ��ʽ��HOOCCH2CH��NH2��COOH��
��1��AΪCH3OOCCH=CHCOOCH3�����е�̼̼˫��C�ܷ����ӳɡ��Ӿۡ������ȷ�Ӧ�����ܷ���������Ӧ��
�ʴ�Ϊ���٢ۢܣ�
��2��BΪHOOCCH=CHCOOH�����еĹ�����Ϊ̼̼˫�����Ȼ����ʴ�Ϊ��̼̼˫�����Ȼ���
��3��������������֪��B�Ľṹ��ʽ�ǣ�HOOCCH=CHCOOH��B�ľ�����ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ�ǣ�CH2=C��COOH��2��
�ʴ�Ϊ��HOOCCH=CHCOOH��CH2=C��COOH��2��
��4��HOOCCH=CHCOOH��״�����������Ӧ�õ�A����Ӧ����ʽΪ��HOOCCH=CHCOOH+2CH3OH$��_{��}^{Ũ����}$CH3OOCCH=CHCOOCH3+2H2O��
�ʴ�Ϊ��HOOCCH=CHCOOH+2CH3OH$��_{��}^{Ũ����}$CH3OOCCH=CHCOOCH3+2H2O��������Ӧ��
��5�������Ϸ�����֪�����Ŷ�����Ľṹ��ʽ�ǣ�HOOCCH2CH��NH2��COOH���ʴ�Ϊ��HOOCCH2CH��NH2��COOH��
��6�����������Ŷ�����һ�������¿�����������һ����Ԫ���Ľṹ�����ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ��
2 HOOCCH2CH��NH2��COOH$\stackrel{һ��������}{��}$ +2H2O��
+2H2O��
�ʴ�Ϊ��2 HOOCCH2CH��NH2��COOH$\stackrel{һ��������}{��}$ +2H2O��
+2H2O��
���� ���⿼���л�����ƶϣ��ؼ��Ǹ���A�����ʼ�B�ķ���ʽ���ṹ�ص�������ۺϷ���ȷ��B�Ľṹ��ʽ����Ŀ�Ѷ��еȣ�

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�| A�� | �����£�pH=1����Һ�л����Դ������ڣ�K+��MnO4-��SO42-��CH3CHO | |
| B�� | ���н϶�Fe3+����Һ�л����Դ������ڣ�Na+��SO42-��SCN-��CO32- | |
| C�� | ̼�������Һ�������NaOH��Һ��Ӧ��Ca2++2HCO3-+2OH-�TCaCO3��+2H2O+CO32- | |
| D�� | ��FeBr2��Һ��ͨ�����������2Fe2++2Br-+2Cl2�T2Fe3++Br2+4Cl- |
| A�� | �ڷ���¯�ͽӴ����У���ʹ�ù����ĸ�ѹ������������������ȼ�������ʺͶ�������Ĵ�����ת���� | |
| B�� |  ��ͼ�ǹ�ҵ��������豸�������ͼ | |
| C�� | �ȼҵ���й��ִ�����֮ĸ������Ҫ��Ӧ�ǵ�⺣ˮ���õ��ռ����������˳�Ϊ�ȼҵ | |
| D�� | �ϳɰ���ҵ�ǹ�ҵ�������ҵ�ƴ����ǰ��ͱ�֤��ǰ�߿�Ϊ�����߹��ṩ�˲�ֹһ��ԭ�� |
| A�� | �������ܵ�������Ҫ�����¼��ַ�ʽ��ֱ��ȼ�ա����ﻯѧת�����Ȼ�ѧת�� | |
| B�� | �������о������۽ṹ�Ĺ����У���ѧ����������������ɨ�������������ֲ�ͬ��εĹ۲������Ⱥ�õ���ʹ�� | |
| C�� | ��Դ�ɷ�Ϊһ����Դ�Ͷ�����Դ����ѧ��ԴҲ�ɷ�Ϊһ�ε�غͶ��ε�� | |
| D�� | ��ײ���ۺ���̬�����dz��õķ�Ӧ�������ۣ����й���̬���ۿɽ����¶ȡ������ȶԷ�Ӧ���ʵ�Ӱ�� |
| A�� | 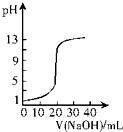 ��ͼ��ʾ0.10 mol•L-1 NaOH��Һ�ζ�20.00 mL 0.10 mol•L-1������Һ�ĵζ����� | |
| B�� | 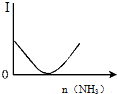 ��ͼ��ʾ������Һ��ͨ�백����������������Һ������I�ı仯 | |
| C�� | 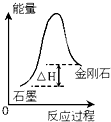 ������ͼ��ʾ��֪��ʯī�Ƚ��ʯ�ȶ� | |
| D�� | 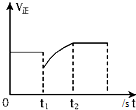 ��ͼ��ʾ��Ӧ2SO2+O2?2SO3��t1ʱ��ֻ��С��SO3��Ũ�� |
| A�� | A��C����Ԫ�ؿ���ɻ�ѧʽΪC2A4�Ļ����� | |
| B�� | E�������ˮ���������� | |
| C�� | F��D�γɵĻ��������ʺܲ����ã������κ��ᷴӦ | |
| D�� | Ԫ�صķǽ�������ǿ������˳����D��C��F��B |