��Ŀ����
11���������ƣ�NaNO2����һ�ֹ�ҵ�Σ�ʵ���ҿ�������װ�ã���ȥ���ּг��������Ʊ���
��֪����2NO+Na2O2=2NaNO2��
��3NaNO2+3HCl=3NaCl+HNO3+2NO��+H2O��
�����������£�NO��NO2������MnO4-��Ӧ����NO3-��Mn2+��Na2O2��ʹ���Ը��������Һ��ɫ��
��1������װ��Aǰ����ͨһ��ʱ��N2��Ŀ�����ų�װ���еĿ�����
��2��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪC+4HNO3��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+4NO2��+2H2O��ʵ�������Bƿ�е���Һ������Ũ������ȴ�ᾧ����������ƣ������˿ɻ��CuSO4•5H2O��
��3������C������Ϊ����ܣ�����ʢ�ŵ�ҩƷΪ��ʯ�ң������ƣ���
��4����ַ�Ӧ����װ��D�в���ķ����ǣ�ȡ�������������Թ��У�����ϡ������Һ�������ݲ��������Թܿ��Ϸ����ֺ���ɫ���壬�������NaNO2��ע���Լ�������
��5��Ϊ�ⶨ�������Ƶĺ�������ȡ4.000g��Ʒ����ˮ���250mL��Һ��ȡ25.00mL��Һ����ƿ�У���0.1000mol•L-1����KMnO4��Һ���еζ���ʵ�������������±���ʾ��
| ����� | 1 | 2 | 3 | 4 |
| KMnO4���/mL | 20.60 | 20.02 | 20.00 | 19.98 |
a����ʽ�ζ���������ˮϴ����δ�ñ�Һ��ϴ
b����ƿϴ����δ����
c���ζ��յ�ʱ���Ӷ���
�ڸ��ݱ������ݣ��������ù������������Ƶ���������86.25%��
��6����ƺ���ʵ��Ƚ�0.1mol•L-1NaNO2��Һ��NO2-��ˮ��̶Ⱥ�0.1mol•L-1HNO2��Һ��HNO2�ĵ���̶���Դ�С������Ҫ˵��ʵ�鲽�衢����ͽ��ۣ�������ҩƷ��ѡ��
25��C��
���� ��1������װ��ͼ��֪�Ʊ�����������Ҫһ�������������Ʒ�Ӧ���ɣ��������ƺͶ�����̼��ˮ����������Ӧ�������Ʊ���һ������������봿�����װ�����������ڣ�
��2��װ��A�з�����Ӧ��Ũ�����̼���ȷ�Ӧ���ɶ�����̼������������ˮ��װ��A�����ɵĶ�����������װ��B�������ᣬ����ͭ��������ͭ������ͭ������Ũ�������������ӷ��Ե��ᣬ������ȴ�ᾧ�õ�����Ϊ����ͭ���壻
��3������CΪ����ܣ��������еļ�ʯ����������һ���������壻
��4����ַ�Ӧ����װ��D�в���ķ���������3NaNO2+3HCl=3NaCl+HNO3+2NO��+H2O����Ӧ���ɵ�һ�������������������ɺ���ɫ��������������飻
��5���ٵ�һ��ʵ���������ĵ����Ը��������Һ���ƫ��
a����ʽ�ζ���������ˮϴ����δ�ñ�Һ��ϴ�����±�Һ��ϡ�ͣ���ҺŨ�ȼ�С��
b���ﵽ��������ƿ����Ҫ�����Ӱ�����Һ�����ʵ����ʵ�����
c���ζ��������Ӷ������������ǰ�Һ���·�������������ʵ�����ĵı�Һ���ƫ��
�ڵ�һ���������������������3�����ı�Һ��ƽ��������ٽ�Ϸ�Ӧ����ʽ�������Ʒ���������Ƶ�����������������
��6������ͬ�¶��°ѵ�Ũ�ȵ��������ƺ�������������ϣ��ⶨ��ҺPH����PH����7˵��ˮ��̶ȴ���PHС��7˵������̶ȴ�
��� �⣺��1���Ʊ�����������Ҫһ�������������Ʒ�Ӧ���ɣ��������ƺͶ�����̼��ˮ����������Ӧ�������Ʊ���һ������������봿�����װ�����������ڣ�����Aǰ����ͨһ��ʱ��N2��Ŀ���ǰ�װ���еĿ����Ͼ����������ɵ��������ƻ������ʣ�
�ʴ�Ϊ���ų�װ���еĿ�����
��2��װ��A�з�����Ӧ��Ũ�����̼���ȷ�Ӧ���ɶ�����̼������������ˮ����Ӧ�Ļ�ѧ����ʽΪ��C+4HNO3��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+4NO2��+2H2O��װ��A�����ɵĶ�����������װ��B�������ᣬ����ͭ��������ͭ������ͭ������Ũ�������������ӷ��Ե��ᣬ������ȴ�ᾧ�õ�����Ϊ����ͭ���壬
�ʴ�Ϊ��C+4HNO3��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+4NO2��+2H2O����ȴ�ᾧ��
��3���Ʊ�����������Ҫһ�������������Ʒ�Ӧ���ɣ��������ƺͶ�����̼��ˮ����������Ӧ�������Ʊ���һ������������봿���������CΪ����ܣ��������еļ�ʯ����������һ���������壬
�ʴ�Ϊ������ܣ���ʯ�ң�
��4��3NaNO2+3HCl=3NaCl+HNO3+2NO��+H2O����Ӧ���ɵ�һ�������������������ɺ���ɫ���������������ַ�Ӧ����װ��D�в���ķ����ǣ�ȡ�������������Թ��м���ϡ������Һ�������ݲ��������Թܿ��Ϸ����ֺ���ɫ���壬֤��������NaNO2��
�ʴ�Ϊ������ϡ������Һ�������ݲ��������Թܿ��Ϸ����ֺ���ɫ���壻
��5���ٵ�һ��ʵ���������ĵ����Ը��������Һ���ƫ�ᵼ�²������ƫ�ߣ�
a����ʽ�ζ���������ˮϴ����δ�ñ�Һ��ϴ�����±�Һ��ϡ�ͣ��ζ����������ĵı�Һ�����ƫ��a��ȷ��
b����ƿ����Ҫ���������ƿϴ����δ�����Ӱ��ⶨ�������b����
c���ζ��������Ӷ��������¶���ƫ������ı�Һ���ƫ��c��ȷ��
�ʴ�Ϊ��ac��
��6�����ڵ�һ������ƫ�ߣ�Ӧ�������������������ı�Һƽ�����Ϊ��$\frac{20.2+20.00+19.98}{3}$mL=20.00mL��
25mL��Ʒ���ĸ�����ص����ʵ���Ϊ0.1000mol/L��0.02L=0.002mol����250mL��Ʒ��Һ�����ĸ�����ص����ʵ���Ϊ0.002mol��$\frac{250ml}{25ml}$=0.02mol�����ݻ��ϼ۱仯�ɵ÷�Ӧ��ϵʽ��2MnO4-��5NO2-����4.000g��Ʒ�к����������Ƶ����ʵ���Ϊ0.02mol��$\frac{5}{2}$=0.05mol������Ϊ69g/mol��0.05mol=3.45g�����Է�Ӧ��Ĺ������������Ƶ���������Ϊ��$\frac{3.45g}{4.000g}$��100%=86.25%��
�ʴ�Ϊ��86.25%��
��6������HNO2��Һ�д��ڵ���ƽ�⣬NO2-����ˮ��ƽ�⣬�Ƚ�0.1mol•L-1NaNO2��Һ��NO2-��ˮ��̶Ⱥ�0.1mol•L-1HNO2��Һ��HNO2�ĵ���̶���Դ�С��ʵ�����Ϊ��25��C��0.1mol/LHNO2��0.1mol/LNaNO2��Һ�������ϣ����ⶨ��ҺPH��7��˵��HNO2�ĵ���̶ȴ���NO2-���ӵ�ˮ��̶ȣ����ⶨ��ҺPH��7��˵��NO2-����ˮ��̶ȴ���HNO2�ĵ���̶ȣ�
�ʴ�Ϊ��25��C��0.1mol/LHNO2��0.1mol/LNaNO2��Һ�������ϣ����ⶨ��ҺPH��7��˵��HNO2�ĵ���̶ȴ���NO2-���ӵ�ˮ��̶ȣ����ⶨ��ҺPH��7��˵��NO2-����ˮ��̶ȴ���HNO2�ĵ���̶ȣ�
���� ���⿼����̽��������ɡ��������ʺ����ķ�������Ŀ�ѶȽϴ��漰����ʵ�鷽������ơ��к͵ζ���������������������ԭ��Ӧ�е���ת�Ƶķ�������ѧ�����֪ʶ����ȷʵ��ԭ������ѧʵ�������������Ϊ���ؼ�������������ѧ���ķ�����������������ѧʵ�顢��ѧ����������

 ��У����ϵ�д�
��У����ϵ�д� W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯��ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Neԭ�ӵĺ�����������l��Y�ĵ�����һ�ֳ����İ뵼����ϣ�Z�ķǽ�������ͬ����Ԫ������ǿ������˵������ȷ���ǣ�������
W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯��ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Neԭ�ӵĺ�����������l��Y�ĵ�����һ�ֳ����İ뵼����ϣ�Z�ķǽ�������ͬ����Ԫ������ǿ������˵������ȷ���ǣ�������| A�� | ��Ӧ�����Ӱ뾶��W��X | |
| B�� | ��Ӧ��̬�⻯����ȶ��ԣ�Y��Z | |
| C�� | ������XZW�Ⱥ����Ӽ����ֺ����ۼ� | |
| D�� | Z���⻯���X������������Ӧˮ�������Һ������Y�������ﷴӦ |
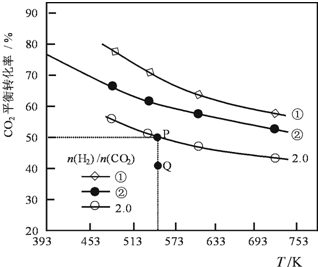 �������ݻ���Ϊ1L�ܱ��������Բ�ͬ����̼��[n��H2��/n��CO2��]����H2��CO2����һ������������Ӧ��2CO2��g��+6H2 ��g��?C2H4��g��+4H2O��g����H��CO2��ƽ��ת�������¶ȵĹ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
�������ݻ���Ϊ1L�ܱ��������Բ�ͬ����̼��[n��H2��/n��CO2��]����H2��CO2����һ������������Ӧ��2CO2��g��+6H2 ��g��?C2H4��g��+4H2O��g����H��CO2��ƽ��ת�������¶ȵĹ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | �÷�Ӧ�ġ�H��0 | |
| B�� | ��̼�ȣ��٣��� | |
| C�� | ����̼��Ϊ2.0ʱ��Q��v��������v���棩 | |
| D�� | ����ʼʱ��CO2��H2Ũ�ȷֱ�Ϊ0.5mol•L-1��1.0mol•L-1����ɵ�P���Ӧ�¶ȵ�ƽ�ⳣ����ֵΪ512 |
| A�� | ��֬������������ˮ�����ɸ�֬������� | |
| B�� | ����ˮ���𱽺������� | |
| C�� | �Ҵ�������͵����ʶ�����������Ӫ������ | |
| D�� | �ϳ�������ά�������л��߷��Ӳ��� |
| A�� | ����ֲ��ͨ�����������տ����еĵ��ǻ�ѧ�仯 | |
| B�� | ��ʯȼ��ȼ��ͨ�����ͷų����������� | |
| C�� | ����β�����ŷŵĵ�����������������̬��ת������ | |
| D�� | ֲ��ո�ȼ��ʱ�ų���������������˵���ѭ�� |
| �������� | ԭ�Ӿ��� | ���Ӿ��� | ���Ӿ��� |
| A�� | ������ | ���� | ������ |
| B�� | ������ | ̼����� | ˮ�� |
| C�� | ���ʯ | �ռ� | �� |
| D�� | �� | ���� | ������ |
| A�� | A | B�� | B | C�� | C | D�� | D |
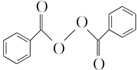 ����ȥ�����������������Ŀǰ�ѱ����ã��ϳɹ�����������������ͼ��ͼ��
����ȥ�����������������Ŀǰ�ѱ����ã��ϳɹ�����������������ͼ��ͼ��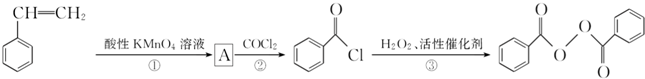
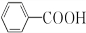 ���ڵķ�Ӧ����Ϊȡ����Ӧ��
���ڵķ�Ӧ����Ϊȡ����Ӧ��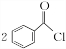 +H2O2$\stackrel{���Դ���}{��}$
+H2O2$\stackrel{���Դ���}{��}$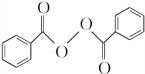 +2HCl��������������������������ˮ��Ļ�ѧ����ʽΪ
+2HCl��������������������������ˮ��Ļ�ѧ����ʽΪ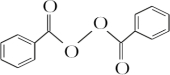 +2H2O$\stackrel{H+}{��}$
+2H2O$\stackrel{H+}{��}$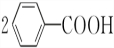 +H2O2��
+H2O2�� ������ˮ��Ӧ���ɱ����ᣬͬ��Ҳ�����Ҵ���Ӧ��д�������Ҵ���Ӧ���ɵ��л���������ƣ�������������
������ˮ��Ӧ���ɱ����ᣬͬ��Ҳ�����Ҵ���Ӧ��д�������Ҵ���Ӧ���ɵ��л���������ƣ�������������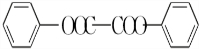 ����
����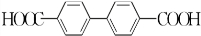 ��
��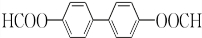 ��
�� ��
��