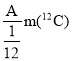��Ŀ����
����Ŀ�������ѡ���(��2����)��Ԫ������Դ�����ϵ�����Ӧ�ù㷺���ش��������⣺
������ͬ���ڵ����и���Ԫ�صĻ�̬ԭ���У��������������̬��ԭ����ͬ��Ԫ����________�֡���̬Ti2+�����������Ų�ʽΪ________________��
����������������Ϊ��I1=580 kJmol-1��I2=1820 kJmol-1��I3=2750 kJmol-1��I4=11600 kJmol-1����������ݹ��ɣ�Ԥ�Ⱶ�������ܵĵ�һ��������ͻԾ���������________֮��(��I1��I2��I3�����![]() ��
��
����֪����A��Ԫ�ص�̼����MCO3�ȷֽ����Ҫ�����ǣ�M2+���̼��������е������ӡ���CaCO3��BaCO3�ķֽ��¶Ƚϸߵ���________________(�ѧʽ)��������________________��
�ȴ���M�ܴ���ϩ����ϩ������ϩ�ȵľۺϣ���ṹ��ͼ1��ʾ��
��M�У�̼ԭ�ӵ��ӻ�������________________��
��M������________![]() ����
����![]() ��
��
A. ���� B. ���� C. ��λ�� D.��� E. ���Ӽ�
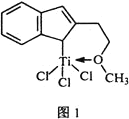
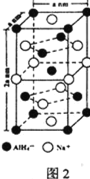
���⻯����(NaAlH4)��һ���������ʴ�����ϣ��侧���ṹ��ͼ2��ʾ��Ϊ�����塣д����AlH4���ռ乹����ͬ��һ�ַ���_______________(�ѧʽ)��NaAlH4�����У���AlH4�������ҵȾ��Na+��________����NaAlH4������ܶ�Ϊ________ gcm-3(�ú�a��NA�Ĵ���ʽ��ʾ)��
���𰸡�4 3s23p63d2 I2��I3 BaCO3 Ca2+�İ뾶С��Ba2+�������CO32���е�O2�������CaCO3�����ֽ� sp2��sp3 DE CH4 8 ![]()
��������
������ͬ���ڵ����и���Ԫ�صĻ�̬ԭ���У��������������̬��ԭ����ͬ��Ԫ�����֡��������������֣���̬Ti�����������Ų�ʽΪ3s23p63d24s2����̬Ti2+�����������Ų�ʽΪ3s23p63d2��
�Ƶ����ܵĵ�һ��������ͻԾ���������I2��I3֮�䡣
�� Ca2+�İ뾶С��Ba2+�������CO32���е�O2����
�Ȣ�M��̼ԭ�ӵŶԵ�����Ϊ0��̼ԭ�ӵ��ӻ�����sp2��sp3����M�У�����������������λ����
��C = Al����AlH4���ռ乹����ͬ��һ�ַ���CH4����AlH4�������ҵȾ��Na+��8��������������ٸ��ݾ�����ܶȵ�����������������м��㡣
������ͬ���ڵ����и���Ԫ�صĻ�̬ԭ���У��������������̬��ԭ����ͬ��Ԫ�����֡��������������֣���̬Ti�����������Ų�ʽΪ3s23p63d24s2����̬Ti2+�����������Ų�ʽΪ3s23p63d2���ʴ�Ϊ��4��3s23p63d2��
�Ʊ�����ʧȥ2�����Ӵﵽ�ȶ��ṹ�������ܵĵ�һ��������ͻԾ���������I2��I3֮�䣬�ʴ�Ϊ��I2��I3��
����CaCO3��BaCO3�ķֽ��¶Ƚϸߵ���BaCO3��������Ca2+�İ뾶С��Ba2+�������CO32���е�O2�������CaCO3�����ֽ⣻�ʴ�Ϊ��BaCO3��Ca2+�İ뾶С��Ba2+�������CO32���е�O2�������CaCO3�����ֽ⡣
�Ȣ�M��̼ԭ�ӵŶԵ�����Ϊ0���ɼ�������Ϊ3��4��̼ԭ�ӵ��ӻ�����sp2��sp3����M�У�����������������λ����������������Ӽ����ʴ�Ϊ��sp2��sp3��DE��
���⻯����(NaAlH4)��һ���������ʴ�����ϣ��侧���ṹ��ͼ2��ʾ��Ϊ�����壬AlH4���ռ乹����ͬ��һ�ַ���CH4��NaAlH4�����У���AlH4�������ҵȾ��Na+��8�����⻯����(NaAlH4)��������4��NaAlH4��NaAlH4������ܶȵ���������������� ���ʴ�Ϊ��CH4��8��
���ʴ�Ϊ��CH4��8��![]() ��
��

����Ŀ���Ȼ�ѧ����1����֪��C(s)+H2O(l)=CO(g)+H2(g) ��H1=a kJ��mol-1��
2CO(g)+O2(g)=2CO2(g) ��H2=bkJ��moL-1��
2H2(g)+O2(g)=2H2O(l) ��H3=ckJ��moL-1��
��C(s)+O2(g)=CO2(g) ��H=___(��a��b��c��ʾ)kJ��moL-1��
��2�����ݼ������ݹ���CH4(g)+4F2(g)=CF4(g)+4HF(g)�ķ�Ӧ����H=___��
��ѧ�� | C��H | C��F | H��F | F��F |
����(kJ��mol-1) | 414 | 489 | 565 | 155 |
����Ŀ�����±���ʵ�����������Ľ��Ͳ���������![]()
ʵ����������� | ����Ľ��� | |
A | ��һƬ�������ھƾ������������գ������ۻ��������� | ���������۵��ر�� |
B | �ò������쵼�ܵ�����������ȼ���۲쵽����ʻ�ɫ | ��ͨ�����к�����Ԫ�� |
C | ��ˮ�м��� | �����˼������ʣ��� |
D | ������ı���Ũ��Һ�еμ�����������ˮ����δ�۲쵽��ɫ�������� | ���屽���ܽ��ڹ����ı����� |
A.AB.BC.CD.D