��Ŀ����
A��B��C��D��E��F��GԪ��ԭ����������������֪Bԭ���������3��δ�ɶԵ��ӣ�Cԭ������������������������֮��Ϊ3��4��E��Cͬ���壬F-��D+��A+���ӵİ뾶��С��������AF������Ϊ���壬G�Ļ�̬ԭ�Ӻ���M�ܲ��������ӣ�N�ܲ�ֻ��1�����ӡ�
�ݴ˻ش��������⣺
��1��д��DԪ�ػ�̬ԭ�ӵĺ�������Ų�ʽ ��B��C��E����Ԫ�صĵ�һ�������ɴ�С��˳����(��Ԫ�ط��ű�ʾ)
��2��A��C���γ�1 8���ӷ��ӣ���ˮ��Һ�е��������Ȼ�����Һʱ�д��������ݳ���д���÷�Ӧ�Ļ�ѧ����ʽ
��3��ij����������������Ԫ���е�����Ԫ����ɣ�Ϊ������������������Ҫ�ɷ֣����л�ѧ������Ϊ ���û�����ˮ��Һ�������Ե�ԭ����(�����ӷ���ʽ��ʾ) ��
��4��0.3molG�ĵͼ����������� molB������������Ӧˮ�������Һǡ����ȫ��Ӧ(�軹ԭ����ֻ��BO)��
��5����������ʱ����B2A4Ϊȼ�ϣ�l mol��̬B2A4������C2��ȼ�գ�����B2����̬A2C���ų�534 kJ��������l molҺ̬A2C��ȫ����������44 kJ��������д����̬B2A4��C2��ȼ������B2��Һ̬A2Cʱ���Ȼ�ѧ����ʽ ��
��15�֣�
��1�� ls22s22p63sl (2��) N��O��S (2��)
��2��2H2O2 2H2O+O2�� ��2�֣�
2H2O+O2�� ��2�֣�
��3�����Ӽ��������ԣ����ۼ� ��2�֣� ClO-+H2O HClO+
OH-��2�֣�
HClO+
OH-��2�֣�
��4��1.4 ��2�֣�
��5��N2H4 (g)+O2 (g) =N2 (g)+ 2 H2O(1) ��H= -622KJ/mol (3��)
��������
������������������֪A��B��C��D��E��F��G�ֱ�ΪH��N��O��Na��S��Cl��Cu��
��1����һ������ͬ���ڴ�����Ϊ�������ƣ����ϵ������μ�С��NΪ�����״̬������������N��O��S��
��2��AC�γ�18�������ʣ����Բ���9+9��ȷ��ΪH2O2�������Ȼ������·����ֽ⣬����������ˮ��
��3�������������ԶԸ����ʽ��в²�ΪNaClO��Ȼ�������ص��ƶϣ�
��4��3Cu2O+14HNO3=6Cu(NO3)2+2NO+4H2O����0.3mol��Ӧ������HNO31.4mol��
��5��N2H4 (g)+O2 (g) =N2 (g)+ 2 H2O(g) ��H= -534KJ/mol��
H2O(g)=H2O(l) ��H= -44KJ/mol�����ݸ�˹���ɿ��Լ���õ���Ӧ�ȣ�д���Ȼ�ѧ����ʽ��
���㣺������Ԫ���ƶ�Ϊ����������ԭ�ӽṹ�����ӽṹ��Ԫ�������ɡ�Ԫ�ؼ�������Ȼ�ѧ����ʽ����д��֪ʶ��

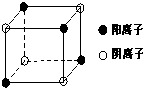 [��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ�
[��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ�