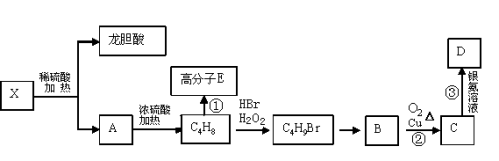
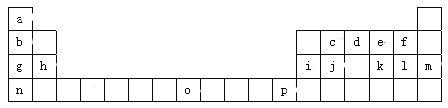
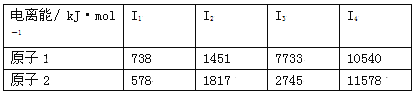
��Ŀ����
����Ŀ��X��Y��Z��M��G����Ԫ�ط������������ڣ���ԭ��������������X��Zͬ���壬���γ����ӻ�����ZX��Y��Mͬ���壬���γ�MY2��MY3���ַ��ӡ��ش��������⣺
��1�� Y��Ԫ�����ڱ��е�λ��Ϊ_____________________________.
��2�� ����Ԫ�ص�����������Ӧ��ˮ����������ǿ����_____________________ (д��ѧʽ)���ǽ�����̬�⻯�ﻹԭ����ǿ����_______________(д��ѧʽ)��
��3�� Y��G�ĵ��ʻ���Ԫ��֮���γɵĻ��������ˮ����������__________________ (д�������������ʵĻ�ѧʽ))��
��4�� ZX�ĵ���ʽΪ_________________��ZX��ˮ��Ӧ�ų�����Ļ�ѧ����ʽΪ__________________��
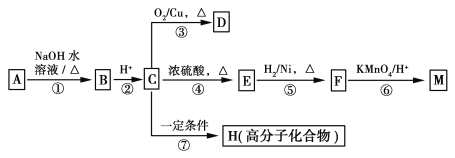
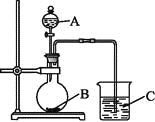
��5�� ����״̬�£�Z�ĵ��ʺ�FeG2����ɿɳ����(װ��ʾ��ͼ����)���ŵ�ʱ����ص�������Ӧ 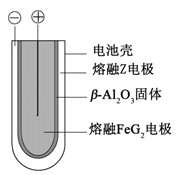
���𰸡���1��Y��2����VIA
��2��HClO4; H2S
��3��O3��Cl2��ClO2��ȡ����������ֶ��ɣ�
��4��![]() ��
��![]()
��5��![]() ��
��
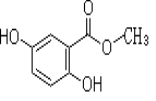
�����������������X��Y��Z��M��G��������Ԫ�ط������������ڣ���ԭ������������������X��HԪ�أ�X��Zͬ���壬���γ����ӻ�����ZX����Zԭ����������Yԭ������������Z��NaԪ�أ�Y��Mͬ���壬���γ�MY2��MY3���ַ��ӣ�����Y��OԪ�أ�M��SԪ�أ�G�Ƕ���������Ԫ�أ�����G��ClԪ�أ�
��1��YΪOԪ�أ���Ԫ�����ڱ��е�λ��Ϊ���ڶ����ڵ���A����2������Ԫ�ص�����������Ӧ��ˮ����������ǿ����HClO4��SԪ�طǽ������������ǽ�����̬�⻯�ﻹԭ����ǿ����H2S��
��3��Y��G�ĵ��ʻ���Ԫ��֮���γɵĻ��������ˮ���������У�Cl2��O3��ClO2�ȣ�
��4�� ZXΪNaH�������ӻ��������ʽΪ![]() ��NaH��ˮ��Ӧ�ų�����Ļ�ѧ����ʽΪ��NaH+H2O=NaOH+H2����
��NaH��ˮ��Ӧ�ų�����Ļ�ѧ����ʽΪ��NaH+H2O=NaOH+H2����
��5�� ����״̬�£�Fe�ĵ��ʺ�FeG2����ɿɳ���أ�Fe�Ǹ������ŵ�ʱ����ص�����������ԭ��Ӧ���缫����ʽ��![]() ��
��

 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д�