��Ŀ����
14������6ƿʧȥ��ǩ��Һ�壬��֪���ǿ������Ҵ������ᡢ����������������֬���������е�һ�֣���ͨ������ʵ����ȷ�����Լ�ƿ����װҺ������ƣ�| ʵ�鲽��ͷ��� | ʵ������ |
| �ٰ�6ƿҺ��ֱ����α��A��B��C��D��E��F��Ȼ������ζ | ֻ��Fû����ζ |
| �ڸ�ȡ�������Թ��У���ˮϡ�� | ֻ��C��D��E���ܽ������ˮ���� |
| �۷ֱ�ȡ����6��Һ�����Թ��У�������Cu��OH�������� | ֻ��Bʹ�����ܽ⣬F�в���ש��ɫ���� |
| �ܸ�ȡC��D��E�������Թ��У���ϡNaOH��Һ������ | ֻ��C���зֲ���������D���Թ����ŵ�������ζ |
��2����D�м���NaOH��Һ�����ȵĻ�ѧ����ʽΪCH3COOCH2CH3+NaOH$\stackrel{��}{��}$CH3COONa+CH3CH2OH��
��3��C�Ķ��ȴ���������ͬ���칹�壻
��4������1mol�Ҵ������е��ǻ�����18O��ǣ���Ũ��������²����������������ַ�Ӧ�������ɵ�������������������88g������ڣ�С�ڻ���ڣ�
��5����֪��ȩ�����������ķ���ʽΪ��CH3CHO+2Ag��NH3��2OH$\stackrel{����}{��}$CH3COONH4+H2O+2Ag��+3NH3����д�������Ƿ���������Ӧ�Ļ�ѧ����ʽCH2OH��CHOH��4CHO+2Ag��NH3��2OH$\stackrel{ˮԡ����}{��}$CH2OH��CHOH��4COONH4+2Ag��+H2O+3NH3��
���� ��ʵ��ٿ�֪��ֻ��FҺ��û����ζ����FΪ��������Һ����ʵ��ۿ�֪��F�в���ש��ɫ������FΪ�����ǣ�ֻ��Bʹ�����ܽ⣬BΪ�����ʵ��ڿ�֪��ֻ��C?E?D����Һ�岻�ܽ������ˮ���ϣ���C��E��DΪ����������������֬����AΪ�Ҵ�����ʵ��ܿ�֪��C���зֲ���������D���Թ����ŵ�������ζ����CΪ����DΪ����������EΪ��֬���Դ������
��� �⣺��ʵ��ٿ�֪��ֻ��FҺ��û����ζ����FΪ��������Һ����ʵ��ۿ�֪��F�в���ש��ɫ������FΪ�����ǣ�ֻ��Bʹ�����ܽ⣬BΪ�����ʵ��ڿ�֪��ֻ��C?E?D����Һ�岻�ܽ������ˮ���ϣ���C��E��DΪ����������������֬����AΪ�Ҵ�����ʵ��ܿ�֪��C���зֲ���������D���Թ����ŵ�������ζ����CΪ����DΪ����������EΪ��֬��
��1��A������Ϊ�ƾ���B�����������ŵ�����Ϊ�Ȼ���D�Ľṹ��ʽΪCH3COOCH2CH3���ʴ�Ϊ���ƾ����Ȼ���CH3COOCH2CH3��
��2��DΪ��������������NaOH��Һ�����ȣ����������ڼ��������·���ˮ�⣬��ѧ��Ӧ����ʽΪCH3COOCH2CH3+NaOH$\stackrel{��}{��}$CH3COONa+CH3CH2OH��
�ʴ�Ϊ��CH3COOCH2CH3+NaOH$\stackrel{��}{��}$CH3COONa+CH3CH2OH��
��3��CΪ�������ȴ������ڡ��䡢������ͬ���칹�壬�ʴ�Ϊ������
��4������CH3COOH+H18OCH2CH3$?_{��}^{ŨH_{2}SO_{4}}$CH3CO18OCH2CH3+H2O����1mol�Ҵ�����������1molCH3CO18OCH2CH3��������Ϊ1mol��90g/mol=90g��88g���ʴ�Ϊ�����ڣ�
��5��������Ϊ���ǻ�ȩ��ȩ�����������Ȼ�����Ӧ�Ļ�ѧ����ʽΪCH2OH��CHOH��4CHO+2Ag��NH3��2OH$\stackrel{ˮԡ����}{��}$CH2OH��CHOH��4COONH4+2Ag��+H2O+3NH3��
�ʴ�Ϊ��CH2OH��CHOH��4CHO+2Ag��NH3��2OH$\stackrel{ˮԡ����}{��}$CH2OH��CHOH��4COONH4+2Ag��+H2O+3NH3��
���� ���⿼���л�����ƶϣ�Ϊ��Ƶ���㣬�����л���Ĺ����������ʡ��л���Ӧ�������Ϊ���Ĺؼ������ط������ƶ��������ۺϿ��飬��Ŀ�ѶȲ���

| A�� | �����ӵ���������6.02��1023 | |
| B�� | ���ʵ�����һ����������ʾ����һ����Ŀ���ӵļ��� | |
| C�� | Ħ�����߸�����������֮һ | |
| D�� | Ħ���������ʵ����ĵ�λ���������ӵ�������λ |
| A�� |  | B�� |  | C�� |  | D�� |  |
| A�� | ת�Ƶ���4.8NA�� | B�� | ������������42.56L����״���� | ||
| C�� | ��ԭ������������0.2mol | D�� | ����ԭ�ĵ�ԭ����11.2g |
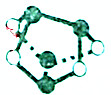
| A�� | 5.6g������0.1mol�����г��ȼ�գ�ת�Ƶ�����Ϊ0.3NA | |
| B�� | 12.5mL 16mol•L-1Ũ����������ͭ��Ӧ��ת�Ƶ�����Ϊ0.2NA | |
| C�� | 7.8g Na2S��Na2O2�Ļ�����к��е�������������0.1NA | |
| D�� | 0.5mol�ۻƣ�As4S4���ṹ��ͼ��������NA��As-S�� |
| A�� | ���³�ѹ�£�0.05NA��CO2������ռ�������1.12L | |
| B�� | 1mol ���������е�ԭ����ΪNA | |
| C�� | ���³�ѹ�£�32g O2��34g H2S������Ӹ�����Ϊ1��1 | |
| D�� | 11.2L NH3��������������Ϊ5NA |
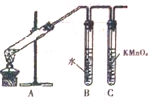 Ϊ̽����������NaOH�Ҵ���Һ������Ӧ�����ɵ��������Ƿ���������װ����ͼ��ʾ���ش�
Ϊ̽����������NaOH�Ҵ���Һ������Ӧ�����ɵ��������Ƿ���������װ����ͼ��ʾ���ش� ����ѧ������Ϊ���ۼ���
����ѧ������Ϊ���ۼ���