��Ŀ����
5��������C��H��O���л���3.0gװ��Ԫ�ط���װ�ã�ͨ��������O2ʹ֮��ȫȼ�գ������ɵ���������ͨ��װ��CaCl2��A���ͼ�ʯ�ң�B���ĸ���ܣ����A������������1.8g��B������������4.4g����֪���л������Է�������Ϊ60����1��ȼ�մ��л���3.0g������O2����״��������L��
��2������л���ķ���ʽ��
��3�������л�����һ�������������Ƶ�������ͭ������Ӧ����д�����л�����ܵĽṹ��ʽ��
���� ��1�����������غ�������������������������������������������
��2��A����������1.8gΪ����ˮ��������B����������4.4gΪ���ɶ�����̼������������n=$\frac{m}{M}$�����л��ˮ��������̼�����ʵ���������ԭ���غ�����л��������N��C����N��H�����ٸ����л������Է����������������N��O����ȷ���л������ʽ��
��3�������л�����һ�������������Ƶ�������ͭ������Ӧ��Ӧ����ȩ�����Ȼ���ȩ������������ԭ��Ӧ���Ȼ������кͷ�Ӧ��
��� �⣺��1����ѧ��Ӧ��ѭ�����غ㶨�ɣ�������O2������Ϊ��1.8g+4.4g-3g=3.2g�������ʵ���=$\frac{3.2g}{32g/mol}$=0.1mol�����������=0.1mol��22.4L/mol=2.24L��
��ȼ�մ��л���3.0g������O22.24L��
��2��CaCl2����ˮ��������1.8g��ˮ�����ʵ���=$\frac{1.8g}{18g/mol}$=0.1mol��n��H��=0.2mol��
��ʯ����CO2����4.4g��������̼���ʵ���=$\frac{4.4g}{44g/mol}$=0.1mol��n��C��=0.1mol
���л������ʵ���Ϊ $\frac{3g}{60g/mol}$=0.05mol��
������������N��C��=$\frac{0.1mol}{0.05mol}$=2��N��H��=$\frac{0.2mol}{0.05mol}$=4��N��O��=$\frac{60-12��2-4}{16}$=2��
�����л���ķ���ʽΪ��C2H4O2��
���л���ķ���ʽΪC2H4O2��
��3�������л�����һ�������������Ƶ�������ͭ������Ӧ��Ӧ����ȩ�����Ȼ���ȩ������������ԭ��Ӧ���Ȼ������кͷ�Ӧ����Ӧ��ͬ���칹���ΪHOCH2CHO��HCOOCH3��CH3COOH��
�𣺸��л�����ܵĽṹ��ʽHOCH2CHO��HCOOCH3��CH3COOH��
���� ���⿼���л������ʽ��ȷ������Ŀ�Ѷ��еȣ�����ȼ�շ������غ�ȷ���л���ķ���ʽ�����ض�ѧ������֪ʶ�Ĺ��̺�ѵ����ּ�ڿ���ѧ��������û���֪ʶ���ʵ�������������

 �˹���������ܹ�����̫���ܣ���CO2��H2O�Ʊ���ѧԭ�ϣ���ͼ��ͨ���˹���������Ʊ�HCOOH��ԭ��ʾ��ͼ������˵����ȷ���ǣ�������
�˹���������ܹ�����̫���ܣ���CO2��H2O�Ʊ���ѧԭ�ϣ���ͼ��ͨ���˹���������Ʊ�HCOOH��ԭ��ʾ��ͼ������˵����ȷ���ǣ�������| A�� | �ù��̿���ʵ��̫��������ܵ�ֱ��ת�� | |
| B�� | ����a���淢��������Ӧ����O2���� | |
| C�� | ����b����������ǿ������a�������Լ��� | |
| D�� | ����a����ķ�Ӧ��CO2+2H++2e-�THCOOH |
| A�� | ������ˮ�������ܣ������ѻ�������ȡ��ˮ�е��� | |
| B�� | ������ˮ�����������������壬������ˮ�ľ����� | |
| C�� | Na2CO3��Һ���м��ԣ������ȵ�ŨNa2CO3��Һϴ���Թ��ڱڵ����� | |
| D�� | Mg��OH��2���ȷֽ�����H2O��MgO�ҡ�H��0������Mg��OH��2����ȼ�� |
| Ԫ�ش��� | X | Y | Z | W |
| ԭ�Ӱ뾶/pm | 160 | 143 | 102 | 74 |
| ��Ҫ���ϼ� | +2 | +3 | +6��+4��-2 | -2 |
| A�� | Y������������Ӧ��ˮ����������ϡ��ˮ | |
| B�� | X��Y�Ľ�����X��Y | |
| C�� | ��̬�軯����ȶ���H2Z��H2W | |
| D�� | ZW3ͨ���õ���Z�뵥��W��Ӧ�Ʊ� |
| A�� | Ԫ�����ڱ���18�� | B�� | ��Ԫ��λ�ڵڶ����ڢ�A�� | ||
| C�� | ��������������������4 | D�� | ����ԭ�Ӱ뾶��С��Ԫ�� |
| A�� | ����Һ̬�����Ƶ��� | |
| B�� | ���罫�����е�N2ת����NO | |
| C�� | ��ҵ�Ϻϳɵ� | |
| D�� | ����ֲ�ォ�����е�N2ת��Ϊ����̬�� |
| A�� | ���ʱ��������������Ӧ | |
| B�� | �ŵ�ʱ�����ĵ缫��ӦΪCH3OH+8OH--6e-�TCO32-+6H2O | |
| C�� | ͨ��0.25mol��������ȫ��Ӧ����1mol����ת�� | |
| D�� | �ŵ�ʱ�������������ƶ� |
| A�� | ���Ȼ�����Һ�еμ�HI��Һ��2Fe3++2HI�T2Fe2++2H++I2 | |
| B�� | ��NH4Al��SO4��2��Һ�е���Ba��0H��2ǡ��ʹ��Ӧ��ȫ��2Ba2++4OH-+Al3++2SO42-�T2BaSO4+AlO2-+2H2O | |
| C�� | 1mol/L��NaAlO2��Һ��2.5 mol/L��HCl��������Ȼ��2AlO2-+5H+�TAl��OH��3+Al3++H2O | |
| D�� | �ù���������ữ�ĺ����ҽ���Һ����ȡ�⣺2I-+H2O2�TI2+2OH- |
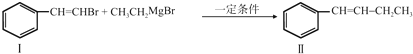
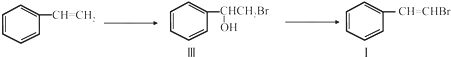
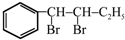 ��
�� ����ע����Ӧ������
����ע����Ӧ������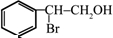 �����Ľṹ��ʽΪ
�����Ľṹ��ʽΪ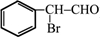 ��
��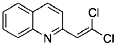 ��BrMgCH2��CH2��3CH2MgBr��һ�������·������Ʒ�Ӧ�ٵķ�Ӧ�����ɵ��л��������������ʽΪC16H17N���Ľṹ��ʽΪ
��BrMgCH2��CH2��3CH2MgBr��һ�������·������Ʒ�Ӧ�ٵķ�Ӧ�����ɵ��л��������������ʽΪC16H17N���Ľṹ��ʽΪ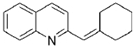 ��
��