��Ŀ����
20���������������ζ������ȩ������ͼ·�ߺϳɣ�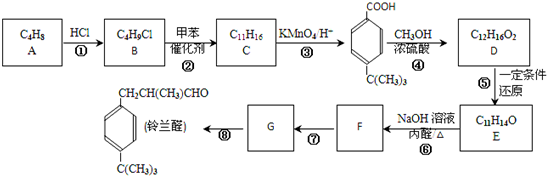
��֪��R1CHO+R2CH2CHO$��_{��}^{NaOH��Һ}$R1CH=
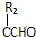
RCl+
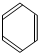 $\stackrel{����}{��}$
$\stackrel{����}{��}$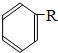 +HCl��R��R1��R2��������
+HCl��R��R1��R2��Ϊ�������밴Ҫ��ش��������⣺
��1��A������2-����ϩ�����춡ϩ������ͼ������ȡ����Ӧ���У��Ӧ��ţ��ڢܣ�
��2��C��ͬϵ�����ʽͨʽ��CnH2n-6��n��6����D�Ĺ����ŵĽṹ��ʽ��-COOR��RΪ��������
��3��д��һ�ּ�������ȩ��ȩ���Ļ�ѧ����ʽ��
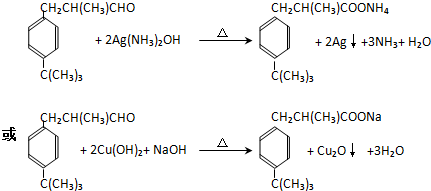 ��
����4����Ӧ���г��и�����M��C17H22O���IJ�����M�Ľṹ��ʽΪ
 ��
����5��д����Ӧ��Ļ�ѧ����ʽ��
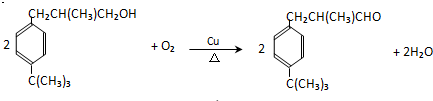 ��
����6��N��D�ķ�����ͬ���칹�壬��D������ͬ�����ţ����ܷ���������Ӧ�������Ͻ���һ��ȡ�������Ҹ�ȡ�����˴Ź���������3�����շ壮д��������������������N��ͬ���칹��Ľṹ��ʽ��
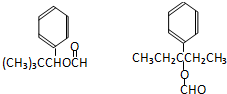 ��
��
���� ��A��B�ķ���ʽ�ж�A��B�ķ�Ӧ�Ǽӳɷ�Ӧ����B��C�ķ���ʽ��֪��B��������Ϣ��ȡ����Ӧ����C����C�����Ը��������Һ�����IJ���Ľṹ�������Ƶ�C�Ľṹ��ʽΪ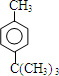 ����������֪AΪCH2=C��CH3��2��BΪ��CH3��3CCl��C����D�ķ�Ӧ��������Ӧ����D�Ľṹ��ʽΪ
����������֪AΪCH2=C��CH3��2��BΪ��CH3��3CCl��C����D�ķ�Ӧ��������Ӧ����D�Ľṹ��ʽΪ ��E��F����������Ϣ�еļӳɷ�Ӧ���������ȩ�Ľṹ����֪F�Ľṹ��ʽΪ
��E��F����������Ϣ�еļӳɷ�Ӧ���������ȩ�Ľṹ����֪F�Ľṹ��ʽΪ ������F�Ľṹ�ٱȽ�D��E�ķ���ʽ��D������ԭ��Ӧ����E���ж�E�Ľṹ��ʽΪ
������F�Ľṹ�ٱȽ�D��E�ķ���ʽ��D������ԭ��Ӧ����E���ж�E�Ľṹ��ʽΪ ��F������ȩ��ת����̼̼˫������������F�����������ӳɷ�Ӧ����GΪ
��F������ȩ��ת����̼̼˫������������F�����������ӳɷ�Ӧ����GΪ ��G�ٷ�����������������ȩ���ݴ˷������
��G�ٷ�����������������ȩ���ݴ˷������
��� �⣺��A��B�ķ���ʽ�ж�A��B�ķ�Ӧ�Ǽӳɷ�Ӧ����B��C�ķ���ʽ��֪��B��������Ϣ��ȡ����Ӧ����C����C�����Ը��������Һ�����IJ���Ľṹ�������Ƶ�C�Ľṹ��ʽΪ ����������֪AΪCH2=C��CH3��2��BΪ��CH3��3CCl��C����D�ķ�Ӧ��������Ӧ����D�Ľṹ��ʽΪ
����������֪AΪCH2=C��CH3��2��BΪ��CH3��3CCl��C����D�ķ�Ӧ��������Ӧ����D�Ľṹ��ʽΪ ��E��F����������Ϣ�еļӳɷ�Ӧ���������ȩ�Ľṹ����֪F�Ľṹ��ʽΪ
��E��F����������Ϣ�еļӳɷ�Ӧ���������ȩ�Ľṹ����֪F�Ľṹ��ʽΪ ������F�Ľṹ�ٱȽ�D��E�ķ���ʽ��D������ԭ��Ӧ����E���ж�E�Ľṹ��ʽΪ
������F�Ľṹ�ٱȽ�D��E�ķ���ʽ��D������ԭ��Ӧ����E���ж�E�Ľṹ��ʽΪ ��F������ȩ��ת����̼̼˫������������F�����������ӳɷ�Ӧ����GΪ
��F������ȩ��ת����̼̼˫������������F�����������ӳɷ�Ӧ����GΪ ��G�ٷ�����������������ȩ��
��G�ٷ�����������������ȩ��
��1����������ķ�����֪��AΪCH2=C��CH3��2��A������Ϊ2-����ϩ �����춡ϩ��������ȡ����Ӧ���Тڢܣ�
�ʴ�Ϊ��2-����ϩ �����춡ϩ�����ڢܣ�
��2��CΪ ��C��ͬϵ�����ʽͨʽΪCnH2n-6��n��6����D�Ľṹ��ʽΪ
��C��ͬϵ�����ʽͨʽΪCnH2n-6��n��6����D�Ľṹ��ʽΪ ��D�Ĺ����ŵĽṹ��ʽΪ-COOR��RΪ��������
��D�Ĺ����ŵĽṹ��ʽΪ-COOR��RΪ��������
�ʴ�Ϊ��CnH2n-6��n��6����-COOR��RΪ��������
��3��������������Һ������������ͭ����Һ��������ȩ�е�ȩ������Ӧ�Ļ�ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��4��E��Gת���Ĺ����У������з���ʽΪC17H22O�ĸ�����M��������ΪG�������Դ���ȩ�����������ȩ��������������֪�ٵķ�Ӧ������M�Ľṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��5����Ӧ��Ļ�ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��6��D�Ľṹ��ʽΪ ��N��D�ķ�����ͬ���칹�壬��D������ͬ�����ţ����ܷ���������Ӧ��˵�������������������Ͻ���һ��ȡ�������Ҹ�ȡ�����˴Ź���������3�����շ壬˵����ȡ������������λ�õ���ԭ�ӣ��������������������N�Ľṹ��ʽΪ
��N��D�ķ�����ͬ���칹�壬��D������ͬ�����ţ����ܷ���������Ӧ��˵�������������������Ͻ���һ��ȡ�������Ҹ�ȡ�����˴Ź���������3�����շ壬˵����ȡ������������λ�õ���ԭ�ӣ��������������������N�Ľṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л����ƶϣ�Ϊ��Ƶ���㣬���ؿ���ѧ�������ƶϼ�֪ʶǨ����������������ͼ�����ʵĽṹ��ʽ������ʽ����Ӧ������������Ϣ���������ϵķ��������ƶϣ���Ϥ�����л���Ӧ���ͼ���Ӧ�������ѵ��ǣ�6����ͬ���칹��������Ӧ����ʽ����д����Ŀ�Ѷ��еȣ�

 ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�| A�� | pH=5��NH4Cl��Һ�������Һ�У���ˮ�������c��H+����Ϊ10-9mol•L-1 | |
| B�� | �����£���pH=3�Ĵ����pH=11��NaOH��Һ�������Ϻ�pH��7 | |
| C�� | ��c��H+����c��OH-��=1��l012����Һ�У�Na+��I-��NO3-��SO42-�ܴ������� | |
| D�� | 0.1 mol•L-1 Na2CO3��Һ��0.1 mol•L-1 NaHSO4��Һ�������ϣ���Һ�У�c��Na+��+c��H+��=c��CO32-��+c��SO42-��+c��HCO3-��+c��OH-�� |
| ѡ�� | ������ | ������ |
| A | SO2��Ư���� | SO2��ʹ��ˮ�����Ը��������Һ��ɫ |
| B | Fe3+��ǿ������ | FeCl3��Һ�����ڻ��շϾɵ�·���е�ͭ |
| C | Ũ��������ˮ�� | Ũ�����ʹ���Ǻ�ֽ��̼����� |
| D | SiO2�е����� | SiO2�������Ʊ����ά |
| A�� | A | B�� | B | C�� | C | D�� | D |
��1��Pλ��Ԫ�����ڱ��������ڵڢ�A�壻Cr�Ļ�̬ԭ�Ӽ۲�����Ų�ʽ��3d54s1��O��һ��18���ӽṹ�⻯��ĵ���ʽ��
 ��
����2���á�������������գ�
| �縺�� | �۷е� | �ȶ��� | ���� |
| O��N | NH3��PH3 | Fe2+�� Fe3+ | H3PO4��HNO3 |
��4����֪��
3Fe��s��+2O2��g��=Fe3O4��s����H=-1118.4kJ/mol
2H2��g��+O2��g��=2H2O��g����H=-438.6kJ/mol��
��3Fe��s��+4H2O��g��=Fe3O4��s��+4H2��g���ġ�H=-151.2KJ/mol��
| A�� | �����ƺ����۳�����ʳƷ����� | |
| B�� | ���������ж����Բ�������ʳƷ������ | |
| C�� | ̼�ᱵ������ˮ�����ҽ������������ | |
| D�� | ������ͨ��ϡ����ʱ�ܲ��������ЧӦ |
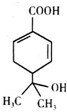
| A�� | ����ʽΪC10H13O3 | |
| B�� | �������ֹ����ţ���ʹ��ˮ�����Ը��������Һ��ɫ | |
| C�� | �ܷ����ӳɡ�ȡ���������ȷ�Ӧ | |
| D�� | ���ƺ�����������Һ���ܷ�����Ӧ���ҵõ��IJ�����ͬ |
 ��
�� ��
�� ��
��
