��Ŀ����
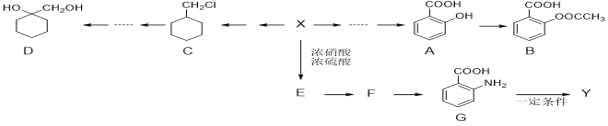
����Ŀ������������Ͷ���������������������Ҫ���ʣ���ҵ�ö��ַ�����������ij���ۺϴ�����NH4����ˮ��ҵ����(��Ҫ��NO��CO��CO2��SO2��N2)��������ͼ��
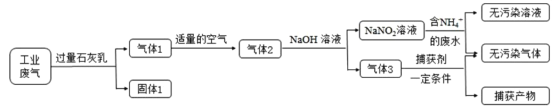
��֪��NO+NO2+2NaOH=2NaNO2+H2O 2NO2+2NaOH=NaNO3+NaNO2+H2O
(1)����1����Ҫ�ɷ���Ca(OH)2��_______(�ѧʽ)��
(2)��NaNO2��Һ������NH4����ˮ��Ӧ�����ӷ���ʽΪ____��
(3)��֤��ˮ��NH4���ѻ��������ķ�����________(д�����������������)��
(4)����1ת��Ϊ����2ʱ�������ܹ�����ԭ����_________��
(5)����������������Ҫ��__________(�ѧʽ)��
(6)���������ɵ�NaNO2����ۺ�ʳ�����ƣ�������ζ������ʹ����ʳ�ж�����֪NaNO2�ܷ������·�Ӧ��2NaNO2+4HI=2NO��+I2+2NaI+2H2O��I2����ʹ���۱���������������Ӧ��ѡ�������г��������ʺ��й��Լ�����ʵ�飬�Լ���NaNO2��NaCl����ѡ�õ�������____(�����)��
��ˮ �ڵ��۵⻯����ֽ �۵��� �ܰ� �ݰ״�
A���٢ۢ� B���٢ڢ� C���٢ڢ� D���٢ڢۢ�
���𰸡�CaCO3��CaSO3 NH4++NO2-=N2��+2H2O ȡ�����������ˮ���Թ��У�����NaOH��Һ���ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ����������������֤��NH4���ѻ������� ����1ת��Ϊ����2ʱ��ֻ�е�����NO��NO2���ʵ���֮��Ϊ1��1ʱ���ſ��Ա�NaOH��Һ��ȫת����NaNO2������������������NaOH��Ӧ����NaNO3��NaNO2�Ļ����Һ����˿������ܹ��� CO C
��������
��ҵ������CO2��SO2�ɱ�ʯ�������գ������ΪCaCO3��CaSO3������Ca(OH)2��������Dz��ܱ�����ʯ��ˮ���յ�N2��NO��CO��������������Ŀ������÷�Ӧ����NO2������NaOH��Һ�����õ�NaNO2���������ܹ���������õ�NaNO3���ɢ�2NO+O2=2NO2����NO2+NO+2NaOH=2NaNO2+H2O������+����2�õ�4NO+O2+4NaOH=4NaNO2+4H2O��Ϊȷ����Ӧֻ����NaNO2��������Ӧ����NO��O2���ʵ���֮��4��1������3����CO��N2��NaNO2�뺬��NH4+����Һ��Ӧ��������Ⱦ��N2��������������������Ҫ��CO�����ݵ�����Һ��I2��Ϊ��ɫ����NaNO2�Ĵ��ڡ�
(1)��ҵ������CO2��SO2����������������Ca(OH)2������Ӧ������ɱ�ʯ�������շ�Ӧ������Ӧ���Σ����Թ����ΪCaCO3��CaSO3������Ca(OH)2��
(2)��NaNO2���������ԣ�NH4+���л�ԭ�ԣ���������Һ�з���������ԭ��Ӧ����N2��H2O��������NaNO2��Һ������NH4����ˮ��Ӧ�����ӷ���ʽΪ��NH4++NO2-=N2��+2H2O��
(3)NH4+�ܹ���ǿ��Ȳ��������������������������֤��ˮ��NH4+�ѻ��������ķ����ǣ�ȡ�����������ˮ���Թ��У�����ŨNaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ����������������֤��NH4���ѻ���������
(4)������������NO�ᱻ��ȫ��������NO2��ֻ������Ӧ2NO2+2NaOH=NaNO3+NaNO2+H2O��ʹ��ȡ�õ��������к���NaNO3���ʣ����ݷ���ʽNO+NO2+2NaOH=2NaNO2+H2O��֪��ֻ�е�������NO��NO2���ʵ���֮��Ϊ1��1ʱ�ſ��Ա�NaOH��Һ��ȫת����NaNO2������ͨ��Ŀ������ܹ�����
(5)����3����CO��N2��NaNO2�뺬��NH4+����Һ��Ӧ��������Ⱦ���壬���ɵĸ�����ӦΪN2��������������������Ҫ��CO��
(6)���ݷ�Ӧ����ʽ2NaNO2+4HI=2NO��+I2+2NaI+2H2O��֪��NaNO2���������ԣ��ὫKI��������I2��I2����ʹ���۱�������Ӧ��������Һ�н��У���Ӧ����Һ�н��У���Ҫˮ���ᣬ����ҪKI�����ۣ����Լ���NaNO2��NaCl��Ҫ���Լ����Ϊ�٢ڢݣ��ʺ���ѡ����C��

 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�