��Ŀ����
����Ŀ��ʵ����������ʯ�����Һ����Ҫ��Fe3+������һ������Al3+��Mn2+��Ca2+��Mg2+�ȣ�Ϊԭ���Ʊ��ߴ����������������£�
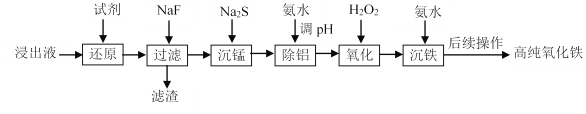
��֪��������ؽ������������������������pH���±���
�������� | Fe3+ | Fe2+ | Al3+ |
��ʼ������pH | 1.1 | 5.8 | 3.0 |
������ȫ��pH | 3.2 | 8.8 | 5.0 |
��1������ԭ��ʱ��Ӧѡ��_____________������ĸ����
A��Zn�� B��NaClO��Һ C��Fe��
��2��������������Ҫ�ɷֳ���������MnF2�⣬������_____________���ѧʽ����
��3�������̡�ʱ����֪��Ksp(MnS)��4.65��10�C14��Ϊȷ��������ȫ[����Һ��c(Mn2��)��1.0��10�C6 mol��L1]��Ӧ������Һ��c(S2-)��_____________mol��L��1��
��4����������ʱ��������ҺpH�ķ�ΧΪ_____________��
��5����������ʱ����������K2Cr2O7��Һ����Ƿ����δ�������Ľ������ӣ��÷�Ӧ�����ӷ���ʽΪ_____________��
��6����������ʱ����Ӧ�¶�Ϊ85�������£���Ӧʱ��ͷ�Ӧ�յ�pH�����ij����ʵ�Ӱ��ֱ�����ͼ��ʾ������ѵĹ���������_____________��
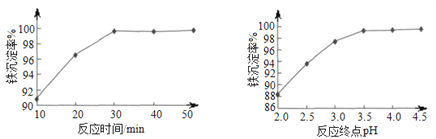
���𰸡� C CaF2 ��MgF2 4.65��10��8 5.0��5.8 Cr2O72-+14H++6Fe2+ �T2Cr3++6Fe3++7H2O ��Ӧʱ��30min ���յ�pH=3.5
�������������������1������ԭ��ʱ����������������
��2����������ͼ������Һ����Al3+��Mn2+��Ca2+��Mg2+ �������������Ƴ�ȥ�����������백ˮ��ȥ�����ӣ����Լ���������dz�ȥCa2+��Mg2+��
��3������![]() ����c(S2-)��Ũ����
����c(S2-)��Ũ����
��4����������ʱ������������ȫ���������������Ӳ��ܳ�����
��5��K2Cr2O7��Һ��Fe2+����ΪFe3+��
��6���ӷ�Ӧ���ʡ��������ʷ�����
��������1��Ϊ������������������Fe3+��ԭΪFe2+ʱ��Ӧѡ�����ۣ���C��ȷ��
��2������Һ����Al3+��Mn2+��Ca2+��Mg2+ �������������Ƴ�ȥ�����������백ˮ��ȥ�����ӣ����Լ���������dz�ȥCa2+��Mg2+ ������������Ҫ�ɷֳ���������MnF2�⣬������CaF2 ��MgF2��
��3������![]() ��
�� ![]() = 4.65��10��8������Ӧ������Һ��c(S2-)��4.65��10��8 mol��L��1��
= 4.65��10��8������Ӧ������Һ��c(S2-)��4.65��10��8 mol��L��1��
��4����������ʱ����������ȫ���������������Ӳ��ܳ��������ݱ������ݣ�������ҺpH�ķ�ΧΪ5.0��5.8��
��5��K2Cr2O7��Һ��Fe2+����ΪFe3+����Ӧ�����ӷ���ʽΪCr2O72-+14H++6Fe2+ �T2Cr3++6Fe3++7H2O��
��6�����ݷ�Ӧ���ʡ��������ʣ���ѵĹ��������Ƿ�Ӧʱ��30min ���յ�pH=3.5��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��CoCl2 6H2O���������������������Ͽ��ú��ܷ��ϣ���ҪΪCo��������Fe��Al�����ʣ�Ϊԭ������ȡCoCl2 6H2O,�������Ʊ������ʵ�һ���¹������̣�

��֪������������������������ʽ����ʱ��Һ��pH���±���
������ | Fe(OH)3 | Fe(OH)2 | Co(OH)2 | Al(OH)3 |
��ʼ���� | 2.7 | 7.5 | 7.6 | 3.8 |
��ȫ���� | 3.2 | 9.7 | 9.2 | 5.2 |
��ش��������⣺
(1)�������ʱCoת��ΪCo2+����Ӧ�����ӷ���ʽΪ__________���������ʱ�����������ԭ����_______��
(2)������������ʹ3 mol��Fe2+ΪFe3+������Ҫ�����������������������Ϊ_________g��
(3)����̼���Ƶ���pH��a��a�ķ�Χ��___________���������������������ֳ����Ļ�ѧʽΪ_________��
(4)��Һ�м������Ŀ����_____________________��
(5)����IΪ___________________��
(6)�� CoCl26H2O��NH4Cl��H2O2Ũ��ˮΪԭ�Ͽ����Ʊ�[Co(NH3)6]Cl3�ķ�Ӧ����ʽΪ__________��