��Ŀ����
����Ŀ������X��Y��Z��W��R��T���ֶ���������Ԫ�أ����ǵ�ԭ�������������� W��Rͬ���壬��W���⻯�ﳣ��ʱΪҺ̬��X��Y������������֮����Z��������������ȣ�X�ֱ���Y��Z��W�γɵ���������ȵķ��ӡ�
���û�ѧ����ش��������⣺
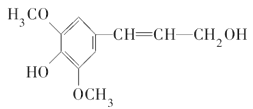
��1��Y��Z��WԪ�ص��⻯��е��ɸߵ��͵�˳��Ϊ____________���û�ѧʽ��ʾ����
��2��ѡȡ��������Ԫ���е�ijЩԪ����ɵĻ������У�д���Ⱥ����Լ��ֺ��Ǽ��Լ�������Է���������С������______________��д����ʽ��������������������������¿ɹ���ȼ�ϵ�أ��õ�طŵ�ʱ�������ķ�ӦʽΪ_______________________________��
��3������һ����ѧ����ʽ֤��WԪ�صķǽ�����ǿ��RԪ��_________________________________��
��4����������������ѧ��FuNvio Cacace���˻���˼��������о������Z4��̬���ӡ�Z4���ӽṹ��ͼ��ʾ����֪����lmolZ-Z ����167kJ������������lmol Z![]() Z�ų�942kJ��������д��Z4��̬���ӱ��Z2��̬���ӵ��Ȼ�ѧ����ʽ��_________________________________��
Z�ų�942kJ��������д��Z4��̬���ӱ��Z2��̬���ӵ��Ȼ�ѧ����ʽ��_________________________________��

��5������X��Z��W����Ԫ���γɵ�һ�����ӻ������ˮ��Һ������Ũ���ɴ�С��˳����________________________��
��д������û������������ӵ�ʵ�����������_________________________________________��
���𰸡� H2O>NH3>CH4 ![]() C2H2-10e-+4H2O= 2CO2+10H+ O2+2H2S=S��+2H2O N4(g):=2N2(g) ��H=-882kJmol-1 c(NO3��)>c(NH4��)>c(H��)>c(OH��) ȡ������Ʒ����Һ�����Թ��У�����������Ũ������������Һ�����ȣ�������ʹʪ���ɫʯ����ֽ���������壬֤������笠����ӡ�
C2H2-10e-+4H2O= 2CO2+10H+ O2+2H2S=S��+2H2O N4(g):=2N2(g) ��H=-882kJmol-1 c(NO3��)>c(NH4��)>c(H��)>c(OH��) ȡ������Ʒ����Һ�����Թ��У�����������Ũ������������Һ�����ȣ�������ʹʪ���ɫʯ����ֽ���������壬֤������笠����ӡ�
�����������⿼��Ԫ�������ɺ�Ԫ�����ڱ���Ӧ�ã�W���⻯�ﳣ����ΪҺ̬����WΪO��W��Rͬ���壬ԭ����������RΪS��X�ֱ���Y��Z��W�γɵ���������ȵķ��ӣ�����Ƴ�XΪH��X��Y������������֮����Z��������������ȣ�YΪC��ZΪN�������ڶ�����Ԫ�أ����TΪCl����1��NH3��H2O���з��Ӽ������CH4û�з��Ӽ���������CH4�ķе���ͣ�H2O������ΪҺ̬��NH3������Ϊ���壬��˷е�ߵͣ�H2O>NH3>CH4����2�����м��Լ���Ӧ����������Ԫ����ɣ��Ҵ��ڷǼ��Թ��ۼ�������ͬ��Ԫ�ع��ɻ�ѧ������Է���������С��������Ϊ��Ȳ�������ʽΪ![]() ������ԭ��صĹ���ԭ������Ȳ�ڸ�����ʧ���ӣ����������ԣ������缫��ӦʽΪC2H2��4H2O��10e��=2CO2��10H������3������������ԭ��Ӧ���������������Դ�����������������ԣ���2H2S��O2=S����2H2O����4���˷�Ӧ��N4=2N2��1molN4�к���6molN��N�����ݷ�Ӧ������ܵĹ�ϵ����H=(6��167��2��942)kJ��mol��1=��882kJ��mol��1������Ȼ�ѧ��Ӧ����ʽΪ��N4(g)=2N2(g) ��H=-882kJ��mol��1����5����������ӻ�������NH4NO3������ǿ�������Σ���Һ�����ԣ�����Ũ�ȴ�С˳���ǣ�c(NO3��)>c(NH4��)>c(H��)>c(OH��)���ڸû������е���������NH4��������ʱһ��ת����NH3�����岽���ǣ�ȡ������Ʒ����Һ�����Թ��У�����������Ũ������������Һ�����ȣ�������ʹʪ���ɫʯ����ֽ���������壬֤������笠�������
������ԭ��صĹ���ԭ������Ȳ�ڸ�����ʧ���ӣ����������ԣ������缫��ӦʽΪC2H2��4H2O��10e��=2CO2��10H������3������������ԭ��Ӧ���������������Դ�����������������ԣ���2H2S��O2=S����2H2O����4���˷�Ӧ��N4=2N2��1molN4�к���6molN��N�����ݷ�Ӧ������ܵĹ�ϵ����H=(6��167��2��942)kJ��mol��1=��882kJ��mol��1������Ȼ�ѧ��Ӧ����ʽΪ��N4(g)=2N2(g) ��H=-882kJ��mol��1����5����������ӻ�������NH4NO3������ǿ�������Σ���Һ�����ԣ�����Ũ�ȴ�С˳���ǣ�c(NO3��)>c(NH4��)>c(H��)>c(OH��)���ڸû������е���������NH4��������ʱһ��ת����NH3�����岽���ǣ�ȡ������Ʒ����Һ�����Թ��У�����������Ũ������������Һ�����ȣ�������ʹʪ���ɫʯ����ֽ���������壬֤������笠�������

 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�����Ŀ��ʵ����������ʯ�����Һ����Ҫ��Fe3+������һ������Al3+��Mn2+��Ca2+��Mg2+�ȣ�Ϊԭ���Ʊ��ߴ����������������£�

��֪��������ؽ������������������������pH���±���
�������� | Fe3+ | Fe2+ | Al3+ |
��ʼ������pH | 1.1 | 5.8 | 3.0 |
������ȫ��pH | 3.2 | 8.8 | 5.0 |
��1������ԭ��ʱ��Ӧѡ��_____________������ĸ����
A��Zn�� B��NaClO��Һ C��Fe��
��2��������������Ҫ�ɷֳ���������MnF2�⣬������_____________���ѧʽ����
��3�������̡�ʱ����֪��Ksp(MnS)��4.65��10�C14��Ϊȷ��������ȫ[����Һ��c(Mn2��)��1.0��10�C6 mol��L1]��Ӧ������Һ��c(S2-)��_____________mol��L��1��
��4����������ʱ��������ҺpH�ķ�ΧΪ_____________��
��5����������ʱ����������K2Cr2O7��Һ����Ƿ����δ�������Ľ������ӣ��÷�Ӧ�����ӷ���ʽΪ_____________��
��6����������ʱ����Ӧ�¶�Ϊ85�������£���Ӧʱ��ͷ�Ӧ�յ�pH�����ij����ʵ�Ӱ��ֱ�����ͼ��ʾ������ѵĹ���������_____________��

����Ŀ��������Ʒ����Ч�ɷּ���;��Ӧ�������
ѡ�� | A | B | C | D |
��Ʒ | ʳ�� | С�մ� | ������������Ƭ | Ư�� |
��Ч�ɷ� | NaCl | Na2CO3 | Al(OH)3 | Ca(ClO)2 |
��; | ����ζƷ | �����ͷ� | ������ҩ | �������� |
A. A B. B C. C D. D