��Ŀ����
14��ij���ӻ�����A�ǻ���ƽ�������ɳɷ�֮һ����Է�������Ϊ117.5���������ֶ����ڵķǽ���Ԫ����ɣ���һ��ǿ��������Ϊȷ��������ɣ���ȡ23.50gAʹ����ȫ�ֽ⣬�������ʵ���֮��Ϊ1��1��2�����ֳ������嵥�ʼס��ҡ�������0.4mol������һ�ֳ����µ�Һ̬��������мͱ��ڷŵ�ʱ���ɵ�B�ڿ��������ɺ���ɫ������C����������ʹƷ����ɫ����1��A�Ļ�ѧʽNH4ClO4��A�������ӵĵ���ʽ
 ��
����2��A�ֽ�Ļ�ѧ����ʽ2NH4ClO4=N2��+Cl2��+2O2��+4H2OA��������ȼ�����ɵ��ʼ����ֳ���������˷�Ӧ�Ļ�ѧ����ʽ��10Al+6NH4ClO4=3N2��+4Al2O3+2AlCl3+12H2O��
��3������C����ˮ������D��D��Ũ��Һ��B�ܣ���ܡ���������Ӧ��ԭ����HNO3�е�+5�۵���NO��+2�۵��ɷ������з�Ӧ��
��4��ʵ���Ҽ���A�������ӵ�ʵ�鷽����ȡ��������A���Թ��У�����������ˮ�ܽ⣬�ټ�������ŨNaOH��Һ���ȣ����Թܿ���һ��ʪ��ĺ�ɫʯ����ֽ���飬����ֽ����ɫ����֤������A�к���NH4+��
���� �����µ�Һ̬��������ˮ������ɫ�������Ƕ����������ͱ�Ϊ������������BΪһ��������������ʹƷ����ɫ��������Ϊ������������A�����ʵ���Ϊ$\frac{23.50g}{117.5g/mol}$=0.2mol�����ɼס��ҡ����������������������������ʵ���Ϊ0.1mol��0.1mol��0.2mol��ˮ������=23.50-0.1��28-0.1��71-0.2��32=7.2g�����ʵ���Ϊ$\frac{7.2g}{18g/mol}$=0.4mol����֪1molA�к���1mol��ԭ�ӣ�1mol��ԭ�ӣ�4mol��ԭ�ӣ�4mol��ԭ�ӣ�A�Ļ�ѧʽΪNH4ClO4��
��� �⣺�����µ�Һ̬��������ˮ������ɫ�������Ƕ����������ͱ�Ϊ������������BΪһ��������������ʹƷ����ɫ��������Ϊ������������A�����ʵ���Ϊ$\frac{23.50g}{117.5g/mol}$=0.2mol�����ɼס��ҡ����������������������������ʵ���Ϊ0.1mol��0.1mol��0.2mol��ˮ������=23.50-0.1��28-0.1��71-0.2��32=7.2g�����ʵ���Ϊ$\frac{7.2g}{18g/mol}$=0.4mol����֪1molA�к���1mol��ԭ�ӣ�1mol��ԭ�ӣ�4mol��ԭ�ӣ�4mol��ԭ�ӣ�A�Ļ�ѧʽΪNH4ClO4��
��1��A�Ļ�ѧʽΪNH4ClO4��NH4+�Ƕ�ԭ�ӹ��ɵ������ӣ�����ʽҪ�������ź͵�ɣ������ʽΪ�� ���ʴ�Ϊ��NH4ClO4��
���ʴ�Ϊ��NH4ClO4�� ��
��
��2��A�ֽ����ɵ�����������������ˮ����ѧ����ʽ��2NH4ClO4=N2��+Cl2��+2O2��+4H2O��NH4ClO4��������ȼ�����ɵ������������������Ȼ�����ˮ����ѧ����ʽ��10Al+6NH4ClO4=3N2��+4Al2O3+2AlCl3+12H2O���ʴ�Ϊ��2NH4ClO4=N2��+Cl2��+2O2��+4H2O��10Al+6NH4ClO4=3N2��+4Al2O3+2AlCl3+12H2O��
��3������C������������ˮ���������ᣬHNO3�е�+5�۵���NO��+2�۵��ɷ������з�Ӧ���ʴ�Ϊ���ܣ�HNO3�е�+5�۵���NO��+2�۵��ɷ������з�Ӧ��
��4���������к���NH4+�ķ����ǣ�ȡ������������NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�����ֽ��������˵�������к���NH4+���ʴ�Ϊ��ȡ��������A���Թ��У�����������ˮ�ܽ⣬�ټ�������ŨNaOH��Һ���ȣ����Թܿ���һ��ʪ��ĺ�ɫʯ����ֽ���飬����ֽ����ɫ����֤������A�к���NH4+��
���� ���⿼�����ʵ��ƶϣ��漰����������ѧ����ʽ����д�����ʵļ���ȣ���Ŀ�Ѷ��еȣ���ѧ����ʽ����д�ǽ�����ѵ㣮

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�| A�� | ������ˮ��̼�����Ʋ���Ӧ | |
| B�� | 25��ʱ��0.1mol/L�Ĵ�����Һ��0.01mol/L�Ĵ�����Һ�У�Kaǰ��С�ں��� | |
| C�� | NaClO��Һ��ͨ������������̼�ķ�ӦΪ��2NaClO+CO2+H2O=Na2CO3+2HClO | |
| D�� | �����ʵ���Ũ�ȵ�̼������Һ����������Һ������������Һ��pH pH��Na2CO3����pH��NaClO����pH��CH3COONa�� |
| A�� | 1 mol•L-1��FeCl3��Һ�У�Fe3+���Ӹ���С��NA | |
| B�� | Al3+��S2-��NO3-��S2-�����������ᡢ���������Һ�о����ܴ������� | |
| C�� | Ca��HCO3��2��Һ�����NaOH��Һ��Ӧ�����ӷ���ʽ��Ca2++HCO3-+OH-=CaCO3��+2H2O | |
| D�� | ��10 mL 0.1 mol•L-1 CH3COONa��Һ�м���5 mL 0.1 mol•L-1����ʱ����Һ��c��CH3COO-����c��CH3COOH����c��H+����c��OH-�� |
| A�� | ����ˮ�������У�����ͨ���۲�������ʧ��ʱ����죬���ж����������ڲ�ͬ������ˮ�����ʵIJ�� | |
| B�� | ̽�����¶�Ӱ�������������ϡ���ᷴӦ���ʵ����ء���ʵ���У���Ҫ�õ��ļ�������ֻ����Ͳ���¶ȼ� | |
| C�� | ���帻�������У����ڷ�Һ©���м���4mL��ˮ���������м���10mL���Ȼ�̼�������á���Һ�����²�Һ�� | |
| D�� | ʵ�����Ʊ���ϩ��������ͭ��Һϴ�� |
 ȫ��Һ�����ܵ�������ò�ͬ��̬���Ӷ�������ԭ��Ӧ��ʵ�ֻ�ѧ�ܺ͵����ת����װ�ã���ԭ����ͼ��ʾ���ŵ�ʱ�����Һ���ɻƱ����������й�˵����ȷ���ǣ�������
ȫ��Һ�����ܵ�������ò�ͬ��̬���Ӷ�������ԭ��Ӧ��ʵ�ֻ�ѧ�ܺ͵����ת����װ�ã���ԭ����ͼ��ʾ���ŵ�ʱ�����Һ���ɻƱ����������й�˵����ȷ���ǣ�������| A�� | ��۵Ķ��Ե缫Ϊ�õ�ص��������Ҳ۵ĵ缫��ӦʽΪV2+-e-=V3+ | |
| B�� | ��������H+���Ҳ۶����ƶ������ | |
| C�� | ���ʱ�����ĵ缫��Ӧʽ VO2++2H++e-=VO2++H2O | |
| D�� | �����ʱ�������Һ��n��H+���ı仯��Ϊ2mol����Ӧת�Ƶĵ�����ΪNA�� |
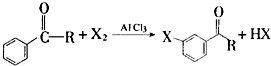


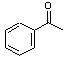 +Br2$\stackrel{AlCl_{3}}{��}$
+Br2$\stackrel{AlCl_{3}}{��}$ +HBr
+HBr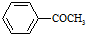 �ķ�������������ͬ���칹�廹��4�֣�������ԭ�ϱ��������������ṹ�б��뺬�б�����
�ķ�������������ͬ���칹�廹��4�֣�������ԭ�ϱ��������������ṹ�б��뺬�б����� �����к˴Ź�������ͼ�й���5�����շ�ķ��ӵĽṹ��ʽΪ
�����к˴Ź�������ͼ�й���5�����շ�ķ��ӵĽṹ��ʽΪ
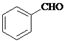 �����Ҵ�Ϊԭ�Ϻϳɱ�������������ͼ
�����Ҵ�Ϊԭ�Ϻϳɱ�������������ͼ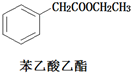 ��
��