��Ŀ����
��֪X��Y��Z��L����Ԫ������ɵ����ʵĻ���Ԫ����ԭ�������������ش��������⣺
��1��L��Ԫ�ط���Ϊ________ ��YԪ��ԭ�Ӻ�������У�δ�ɶԵ�������ɶԵ�����֮��Ϊ______������Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳����____________________����Ԫ�ط��ű�ʾ����
��2��Z��X��Ԫ�ذ�ԭ����Ŀ��l��3�ɹ��ɷ���A��A�ĵ���ʽΪ________��B����Ҳ��Z��X��Ԫ����ɣ���Ϊ���ͷɴ��Ļ��ȼ�ϣ���������һ��Һ̬�������֪�û��������Է�������Ϊ32������XԪ�ص���������Ϊ12��5 %���Ҹ÷��ӽṹ��ֻ�е�������B�ĽṹʽΪ____________����64 g B������Һ̬˫��ˮǡ����ȫ��Ӧ�������������ֲ���Ⱦ��������̬���ʣ����ų�3000 kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ ��
��3������Se��������������Ԫ�أ���Ԫ��Lͬһ���壬�����2 ~5����Ԫ�ص��ʷֱ���H2��Ӧ����l mol��̬�⻯������������������£������ܱ�ʾ����1 mol����������������������__________��ѡ����ĸ��ţ���
a����99��7 kJ b����29��7 kJ c��+20��6 kJ d��+241��8 kJ
��1��O��1��2�� C��N��O��H ��1��1��3�֣�
��2��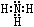 ��1�֣���
��1�֣��� 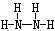 ��1�֣���N2H4(l)+2H2O2(l)��N2(g)+4H2O(g)+1500 kJ��2�֣�
��1�֣���N2H4(l)+2H2O2(l)��N2(g)+4H2O(g)+1500 kJ��2�֣�
��3��b ��1�֣�
���������������1��Y��C Cԭ�ӵĺ�������Ų�ʽ1s22s22p2 ��p�����2��δ�ɶԵ��ӣ��ɶԵ��Ӷ���4��
δ�ɶԵ�������ɶԵ�����֮��Ϊ1:2�� X��H Y:C Z:N L:O ԭ�Ӱ뾶��C��N��O��H ��
��2��Z��N X��H AΪNH3 ����֪�û��������Է�������Ϊ32������XԪ�ص���������Ϊ12��5 %��X��32*0��125/1=4 Z:(32-4)/14=2 B�ķ���ʽ��N2H4 B�Ľṹʽ��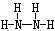 ��64���൱��2mol N2H4�ų�����Ϊ3000 kJ��1mol N2H4�ų�����Ϊ1500 kJ���Ȼ�ѧ����ʽ��N2H4(l)+2H2O2(l)��N2(g)+4H2O(g)+1500 kJ
��64���൱��2mol N2H4�ų�����Ϊ3000 kJ��1mol N2H4�ų�����Ϊ1500 kJ���Ȼ�ѧ����ʽ��N2H4(l)+2H2O2(l)��N2(g)+4H2O(g)+1500 kJ
���㣺������Ԫ���ƶ�Ϊ�������������ʽṹ���Ȼ�ѧ����ʽ��֪ʶ��

 ��У����ϵ�д�
��У����ϵ�д���һ�������£���������Ԫ��R������RO3n����R2���������·�Ӧ��RO3n�� ��2R2�� ��6H����3R��3H2O�����й���Ԫ��R����������ȷ����
| A��Rԭ�ӵ����������4������ | B��RO3n���е�Rֻ�ܱ���ԭ |
| C��R�����������Ų�ʽ��nsnnp2n | D��HnRO4һ����ǿ�� |
��ͼ��������ԭ����ӽ�ʱ�������仯ͼ�����йظ�ͼ��˵����ȷ���ǣ� ��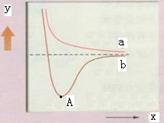
| A��y������ԭ��֮��ĺ˼�� |
| B��x������ϵ���е����� |
| C��a����������������෴��������ԭ�������仯 |
| D��A��ʱ��ʾ��ԭ�Ӽ��γ����ȶ��Ĺ��ۼ� |
����˵����ȷ���ǣ� ��
| A�������ƾ��ǵ����˶�����̬ |
| B����ɫ��Ӧ�������Ƿ������ |
| C��ͭԭ�Ӵ��ڻ�̬ʱ�ĺ�������Ų�ʽΪ3d104s1 |
| D����������˶�״̬������ţ���˶�������������Ҳ������ͳ�Ʒ��������� |
�����±������ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼۣ���Ϣ���ж�����������ȷ����
| Ԫ�ش��� | A | B | C | D | E |
| ԭ�Ӱ뾶��nm | 0��186 | 0��143 | 0��089 | 0��102 | 0��074 |
| ��Ҫ���ϼ� | +1 | +3 | +2 | +6��-2 | -2 |
A������������Ӧˮ����ļ���A>C B���⻯��ķе�H2D < H2E
C��������ϡ���ᷴӦ������A<B D��C2+��A+�ĺ�����������
������Ԫ��Xԭ�Ӻ���ĵ��Ӵ���n�����Ӳ��ϣ�����������Ϊ��2n+1��������������Ϊ��2n2��1�����й�X��˵���в���ȷ����
| A��XԪ����̬�⻯���ˮ��Һ������ |
| B��X���γɻ�ѧʽΪNaXO3�ĺ��������� |
| C��Xԭ�ӵ������������ͺ˵����������Ϊż�� |
| D��XԪ�س������ʵĻ�ѧʽΪX2 |
���ܴ��ڵĵ�119��δ֪Ԫ�أ����˳�Ϊ�����ա�������Ԫ�����ڱ��ṹ�����ʱ仯���ƣ��ж��йء����ա���Ԥ����ȷ����
| A�����з����� | B�������нϸߵ��۵� |
| C���ڻ������г�+2�� | D�����ʵ��ܶ�С��1 g/cm3 |
����˵����ȷ����
| A��ԭ�ӵĵ�һ������Խ��Ԫ�صĵ縺�Ծ�Խ�� |
| B��ԭ�ӵĵ��Ӳ���Խ�࣬ԭ�Ӱ뾶Խ�� |
| C��ԭ��ʧȥ����Խ�࣬˵���仹ԭ��Խǿ |
| D��ͬһԭ�ӵ��ܲ�Խ�ߣ�S�����Ƶİ뾶Խ�� |