��Ŀ����
2�� ij��ѧʵ��С����Ҫ�˽��г���ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ����Ũ�ȣ����ñ�NaOH��Һ��ijƷ��ʳ�ð״��еζ���
ij��ѧʵ��С����Ҫ�˽��г���ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ����Ũ�ȣ����ñ�NaOH��Һ��ijƷ��ʳ�ð״��еζ�����1����ʵ��Ӧѡ�÷�̪��ָʾ��������ƿ����ȡһ������İ״����õ���������ʽ�ζ��ܣ�
��2����ͼ��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����1mL��A���Ŀ̶�Ϊ25���ζ�����Һ�����ӦΪ25.40mL����ʱ�ζ�����Һ����������24.60mL��
��3��Ϊ�˼�Сʵ����һ������������ʵ�飬����ÿ����ȡ�״������ΪVmL����NaOH��Һ��Ũ��Ϊc mol•L-1������ʵ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 26.02 | 25.32 | 25.28 |
A��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱNaOH��Һ�����
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
C��ʢװ��Һ�ĵζ���δ�ñ�Һ��ϴ
D����һ�εζ��õ���ƿ�ô�װҺ��ϴ
E���μ�NaOH��Һ���죬δ������տ�����Һ��ɫ������ֹͣ�ζ�
��4�������������ݣ�д���ð״��д������ʵ���Ũ�ȵı���ʽ�����ػ���$\frac{��25.32+25.28��•c}{2V}$mol/L��
���� ��1��������Ӧ������ǿ�������Σ���Һ�ʼ��ԣ�Ӧѡ����Ա�ɫ��Χ�ڵ�ָʾ���� ��ȡ��Ӧ������ʽ�ζ��ܣ�
��2��A��C�̶ȼ����1ml��˵��ÿ����С��֮����0.1mL��A���Ŀ̶� 25���ݴ�ȷ��B�Ŀ̶ȣ�ע��ζ��ܵ�������ֵС��������ֵ��
��3������c���ᣩ=$\frac{c���•V���}{V���ᣩ}$���жϲ��������������������Ӱ�죻
��4������ƽ��ֵ���������Һ��������ٸ�����ȹ�ʽ���������ʵ���Ũ�ȣ�
��� �⣺��1��������Ӧ������ǿ�������Σ���Һ�ʼ��ԣ�Ӧѡ����Ա�ɫ��Χ�ڵ�ָʾ������ѡ��̪���״����ᣬӦ������ʽ�ζ�����ȡ��
�ʴ�Ϊ����̪����ʽ�ζ��ܣ�
��2��A��C�̶ȼ����1ml��˵��ÿ����С��֮����0.10mL��A���Ŀ̶�Ϊ25��A��B֮�����ĸ�С���������0.40mL����B��25.40mL�����ڵζ���50.00mL�̶��·�����Һ�壬����ʵ����Һ��Һ�����24.60mL��
�ʴ�Ϊ��25.40������24.60mL��
��3�����ϱ����Կ�������һ��ʵ���м�¼����NaOH��Һ������Զ��ں����Σ���õĴ���Ũ��ƫ��
A��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱNaOH��Һ����������������������ƫС�������������Ũ��ƫС���ʴ���
B���ζ�ǰ�ζ��ܼ������ݣ��ζ����������ݣ����������������ƫ�������������Ũ��ƫ����ȷ��
C��ʢװ��Һ�ĵζ���װҺǰ������ˮ��ϴ����δ�ñ�Һ��ϴ��������������Ũ��ƫС���������������������ƫ���������Ũ��ƫ����ȷ��
D����һ�εζ��õ���ƿ�ô�װҺ��ϴ����������δ��ϴ����ϴ��ƿ���´�������ʵ���ƫ������ʹ�õ������������ƫ���������Ũ���жϣ�����ȷ��
�ʴ�Ϊ��BCD��
��4������c���ᣩ=$\frac{c���•V���}{V���ᣩ}$����������ʵ���Ũ��c=$\frac{��25.32mL+25.28mL��•cmol/L}{2VmL}$=$\frac{��25.32+25.28��•c}{2V}$mol/L��
�ʴ�Ϊ��$\frac{��25.32+25.28��•c}{2V}$��
���� ���⿼�����ζ�ʵ�飬��Ŀ�Ѷ��еȣ������ʵ����Ҫѡȡָʾ����ע��ζ����ϵĿ̶Ⱥ���Ͳ�Ͽ̶ȵ�����Ϊ�״��㣮

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д� ���й�����ϩ�ķ�����ȷ���ǣ�������
���й�����ϩ�ķ�����ȷ���ǣ�������| A�� | ����һ�ȴ�����6�� | |
| B�� | ���Ͷ������� ����Ϊͬ���칹�� ����Ϊͬ���칹�� | |
| C�� | һ�������£����ֱ���Է����ӳɡ�ȡ����������Ӧ | |
| D�� | ���ķ��������е�̼ԭ��һ����ͬһ��ƽ���� |
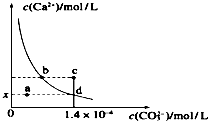 ����ʱ��CaCO3��ˮ�еij����ܽ�ƽ��������ͼ��ʾ����֪CaCO3���ܶȻ������£�Ϊ2.8��10-9������˵���в���ȷ���ǣ�������
����ʱ��CaCO3��ˮ�еij����ܽ�ƽ��������ͼ��ʾ����֪CaCO3���ܶȻ������£�Ϊ2.8��10-9������˵���в���ȷ���ǣ�������| A�� | x����ֵΪ2��10-5 | B�� | c��ʱ��CaCO3���� | ||
| C�� | ��������ˮ��ʹ��Һ��d����a�� | D�� | b����d���Ӧ���ܶȻ���� |
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ����A | 0 |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� | �� | �� |
��1���ڢۡ���Ԫ���У�ԭ�Ӱ뾶������Ca����Ԫ�ط��ţ���
��2���١�����Ԫ������������Ӧ��ˮ������������ǿ����HClO4�������ʻ�ѧʽ���������Ե�����������Al��OH��3�������ʻ�ѧʽ����
��3����Ԫ�ص�����������Ӧˮ���������⻯����������M��M�к��еĻ�ѧ�����������Ӽ������ۼ���
��4���õ���ʽ��ʾԪ�آ�����γɻ�����Ĺ���

��5��д������32�����ӵ�Ԫ�آ��⻯��ķ���ʽC4H8
��6��д����ҵұ���ݵĻ�ѧ����ʽ2Al2O3�����ڣ�$\frac{\underline{\;���\;}}{\;}$4Al+3O2��
��7��Ԫ�آ���������ȼ�յIJ�����ˮ��Ӧ�Ļ�ѧ����ʽ2Na2O2+2H2O=4NaOH+O2����
��S����б��s��+O2��g��=SO2��g����H1=-297.16kJ•mol-1
��S��������s��+O2��g��=SO2��g����H2=-296.83kJ•mol-1
��S�����s��=S��������s����H3��������
| A�� | ��H3=0.33 kJ•mol-1 | |
| B�� | ��б��ת��Ϊ������ķ�Ӧ�����ȷ�Ӧ | |
| C�� | ������ȵ�б���ȶ� | |
| D�� | ��б����������ȶ� |
| A�� | �������մɡ�ˮ�������������β��ϣ�������ԭ�϶���ʯ��ʯ | |
| B�� | �����������̼�ڳ�ʪ�Ŀ�����������ԭ��ض������绯ѧ��ʴ | |
| C�� | ����װ�����绯�˵�̺�����ϰ塢�����ҵȾ����ͷų���Ⱦ�����ļ�ȩ���� | |
| D�� | ������ϩ�������ճ������п���������ʳƷ��װ |
| A�� | �κ�״̬�£�1molCO2��18��ˮ�����ķ�������ԭ��������� | |
| B�� | 22.4L���κ���������ʵ���Ϊ1mol | |
| C�� | �DZ�״���£�1mol�κ����������ض�����22.4L | |
| D�� | ��״���£�1molSO3�������22.4L |
| ѡ�� | ���� | Ӧ�� |
| A | ��֬��һ���������ܷ���ˮ�ⷴӦ | ��֬�ڼ���������ˮ���Ʒ��� |
| B | ����淋����� ��ʹ�����ʱ��� | �����������ɱ�������� |
| C | ����ܷ���ˮ�ⷴӦ | ���õ��ۡ���ά��ˮ���������� |
| D | ��������ˮ�ɷ���ˮ�ⷴӦ | ��������������ˮ�ľ�����ɱ������ |
| A�� | A | B�� | B | C�� | C | D�� | D |