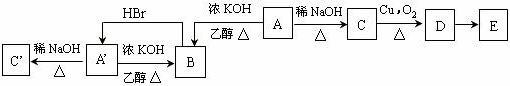
��Ŀ����
4��������Ԫ��W��X��Y��Z��ԭ������������������W�������ӵĺ������������������Ԫ��ԭ�ӵ��ڲ��������ͬ��X��Y������Z�ֱ��γ�ԭ�Ӹ�����Ϊ1��1��1��2����̬�����������������������˵������ȷ���ǣ�������| A�� | ������̬���������������ԭ��������������Ϊ8 | |
| B�� | X��N��Z������W�γɼ��м��Թ��ۼ����зǼ��Թ��ۼ��Ļ����� | |
| C�� | ������W4XY2Z���������ӻ����Ҳ�����ǹ��ۻ����� | |
| D�� | �е㣺W2Z��YW3��XW4 |
���� ������Ԫ��W��X��Y��Z��ԭ��������������W�������ӵĺ������������������Ԫ��ԭ�ӵ��ڲ��������ͬ�������������������������ȶ��ṹ�����ڶ�����Ԫ��ԭ���ڲ������Ϊ2��8������֪W�����Ӻ��������Ϊ2����WΪHԪ�أ�X��Y��Z�����ڵڶ����ڣ�X��Y������Z�ֱ��γ�ԭ�Ӹ�����Ϊ1��1��1��2����̬���������֪XΪC��YΪN��ZΪO��CԪ������Ԫ���γ�C2H2��C2H4��NԪ����OԪ���γ�NO2��N2O4���ݴ˽��
��� �⣺������Ԫ��W��X��Y��Z��ԭ��������������W�������ӵĺ������������������Ԫ��ԭ�ӵ��ڲ��������ͬ�������������������������ȶ��ṹ�����ڶ�����Ԫ��ԭ���ڲ������Ϊ2��8������֪W�����Ӻ��������Ϊ2����WΪHԪ�أ�X��Y��Z�����ڵڶ����ڣ�X��Y������Z�ֱ��γ�ԭ�Ӹ�����Ϊ1��1��1��2����̬���������֪XΪC��YΪN��ZΪO��CԪ������Ԫ���γ�C2H2��C2H4��NԪ����OԪ���γ�NO2��N2O4��
A��������������֪��CԪ������Ԫ���γ�C2H2��C2H4�к���HԪ�أ�Hԭ��һ��������8���ӽṹ����A����
B��CԪ����HԪ���γ�C2H6�ȣ�NԪ����HԪ�ؿ����γ�N2H4��OԪ����HԪ�ؿ����γ�H2O2�����м��Թ��ۼ����зǼ��Թ��ۼ�����B��ȷ��
C��������H4CN2O������ΪNH4CNO������ΪCO��NH2��2��ǰ���������ӻ�����������ڹ��ۻ������C��ȷ��
D��������ˮΪҺ̬�������顢����Ϊ���壬��ˮ�ķе���ߣ�����������֮�����������е���ڼ��飬�ʷе㣺H2O��NH3��CH4����D��ȷ��
��ѡA��
���� ���⿼��ṹ����λ�ù�ϵӦ�ã��ƶ�Ԫ���ǽ���ؼ���A��ע�⺬��Hԭ�ӵķ���һ�����������������ԭ������8���ӽṹ��Cѡ��Ϊ�״��㡢�ѵ㣬ѧ����HCNO���Ϥ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ����һ��ͨʽ��RCOOR���R�������ͬ��Ҳ���Բ�ͬ | |
| B�� | RCOOR���R�������֬��������Ҳ�����Ƿ������� | |
| C�� | ��RCOOR���У���R��R���Ϊ-CH3ʱ���������м������ | |
| D�� | ̼ԭ������ͬ�ı���һԪ����ͱ���һԪ����Ϊͬ���칹�� |
| A�� | �������Һ�л���ˮ����Ӱ���ط�Ӧ | |
| B�� | ��Ӧ�У�SOCl2�����ܼ������������� | |
| C�� | ��ع��������У��������ȣ�SOCl2�� ����ԭΪLi2SO3 | |
| D�� | ��ع��������У�������ṩ�ĵ��������������������ʵ���֮��Ϊ4��1 |
| A�� | ��pH=4 CH3COOH��Һ��ˮϡ��10������Һ�и�����Ũ�Ⱦ���С | |
| B�� | �� CH3COOH��Һ�ζ������ʵ���Ũ�ȵ�NaOH��Һ��pH=7��V��CH3COOH��Һ����V��NaOH��Һ�� | |
| C�� | ��0.2 mol/L��������Һ�м�������0.1 mol•L-1NH3•H2O��Һ��c��Cl-��+c��OH-��=c��H+��+c��NH3•H2O�� | |
| D�� | �ں�0.1mol NaHSO4��Һ�У�c��H+��=c��SO42-��+c��OH-�� |
| A�� | ��״���£�1.12L1H2��0.2g 2H2������0.1NA������ | |
| B�� | ��ͬѹ��ͬ�µ�����£�������ͬ�������ͳ���������ԭ��������ͬ | |
| C�� | ��״���£�11.2L������ȫȼ�պ����ɵ�CO2������Ϊ3.5NA | |
| D�� | �����£���5.6 g ��Ͷ��������Ũ������ת�Ƶ�����Ϊ0.3 NA |
| A�� | 12.4 g�����к��й��ۼ���Ϊ0.4 NA | |
| B�� | 3Fe+4H2O��g��=Fe3O4+4H2��Ӧ�У���5.6 g Feȫ��ת��ΪFe3O4ʱ������0.3 NA���ӷ���ת�� | |
| C�� | ��1 L 0.1 mol/L̼������Һ����������������0.1 NA | |
| D�� | �ڱ�״���£�22.4 L CH4��18 g H2O�����еĵ�������Ϊ10 NA |
| A�� | FeCl3��Һ�У�K��CH3OH��Br-��NO3- | |
| B�� | �ڰ�ˮ��Һ�У�Al3+��NO3-��Cl-��Ag+ | |
| C�� | ij���������Һ�У�NH4+��Fe3+��NO3-��Cl- | |
| D�� | ��ʹ�����Ժ�ɫ����Һ��K+��Cr2O72-��CH2CH2OH��SO42- |
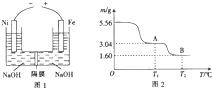 ���������仯�����ڹ�ҵ������������Ҫ��Ӧ�ã�
���������仯�����ڹ�ҵ������������Ҫ��Ӧ�ã�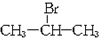 ��
��