��Ŀ����
10��Heck��Ӧ�Ǻϳ�C-C������Ч����֮һ���練Ӧ�٣�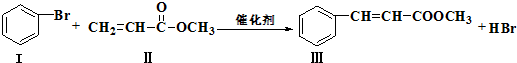
�������������ºϳ�·��ã�
IV������ʽΪC3H6O3��$��_{��}^{ŨH_{2}SO_{4}}$V������ʽΪC3H4O2��$��_{ŨH_{2}SO_{4}}^{CH_{3}OH}$��
��1���������ķ���ʽΪC10H10O2��1mol���������ȫȼ��������Ҫ����11.5mol O2��
��2��������IV���ӽṹ�в�������д��������IV�Ľṹ��ʽ��HOCH2CH2COOH��
д���ɻ�����V��Ӧ���ɻ������Ļ�ѧ����ʽ��CH2=CHCOOH+CH3OH$��_{��}^{Ũ����}$CH2=CHCOOCH3+H2O��
��3���йػ�����I�͢�˵����ȷ���ǣ�BC��
A��1mol ������I ������2.5mol H2�����ӳɷ�Ӧ
B�����������ʹ���Ը��������Һ��ɫ
C��������I������ˮ
D�����������Ӽ�ۺϣ���Ӧ���ɵĸ߾���ṹΪ
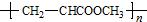 ��
��
���� ��1�����ݻ������Ľṹ��ʽȷ�������ʽ��1molCxHyOz��ȫȼ�պ�����Ϊ��x+$\frac{y}{4}$-$\frac{z}{2}$��mol��
��2�����ķ���ʽΪC3H6O3����Ũ���ᡢ���������µõ�V��Vת���õ����ɢ�Ľṹ��֪��VΪCH2=CHCOOH��������IV���ӽṹ�в�����������Ľṹ��ʽΪHOCH2CH2COOH��
��3��A��������I�б��������������ӳɷ�Ӧ��
B�����������̼̼˫��������ϩ�������ʣ�
C������±������������ˮ��
D�����������Ӽ�ۺ����ɸ߾���Ľṹ��ʽΪ ��
��
��� �⣺��1�����ݻ������Ľṹ��ʽ����֪�����ʽΪC10H10O2��1molC10H10O2��ȫȼ�պ�����Ϊ��10+$\frac{10}{4}$-1��mol=11.5mol��
�ʴ�Ϊ��C10H10O2��11.5��
��2�����ķ���ʽΪC3H6O3����Ũ���ᡢ���������µõ�V��Vת���õ����ɢ�Ľṹ��֪��VΪCH2=CHCOOH��������IV���ӽṹ�в�����������Ľṹ��ʽΪHOCH2CH2COOH���ɻ�����V��Ӧ���ɻ������Ļ�ѧ����ʽ��CH2=CHCOOH+CH3OH$��_{��}^{Ũ����}$CH2=CHCOOCH3+H2O��
�ʴ�Ϊ��HOCH2CH2COOH�� CH2=CHCOOH+CH3OH$��_{��}^{Ũ����}$CH2=CHCOOCH3+H2O��
��3��A��������I�б��������������ӳɷ�Ӧ��1mol ������I ������3mol H2�����ӳɷ�Ӧ����A����
B�����������̼̼˫��������ϩ�������ʣ���ʹ���Ը��������Һ��ɫ����B��ȷ��
C������±������������ˮ����C��ȷ��
D�����������Ӽ�ۺ����ɸ߾���Ľṹ��ʽΪ ����D����
����D����
��ѡ��BC��
���� ���⿼���л�����ƶ���ϳɡ��л���Ľṹ�����ʵȣ�������ѧ���ķ�������������֪ʶǨ�������������Ƕ��л���ѧ���ۺϿ��飬��Ŀ�Ѷ��еȣ�

| A�� | ���ձ��еij�������һ���� | |
| B�� | ���ձ��г�����������ֵ��Ϊ0.078g | |
| C�� | ��Ӧ�����ձ�����Һ��pH���ܣ��ף��� | |
| D�� | ���ձ��з�Ӧ�����ӷ���ʽ��ͬ |
 �����й��ڸ��л������������ȷ���ǣ�������
�����й��ڸ��л������������ȷ���ǣ�������| A�� | ��ʹ����KMnO4��Һ��ɫ | B�� | ��ʹ��ˮ��ɫ | ||
| C�� | ��һ�������¿��Է����Ӿ۷�Ӧ | D�� | һ�������£��ܺ�NaOH����Һ��Ӧ |
| A�� | �������3�����ӵ�Ԫ�� | B�� | �������Ϊ+7�۵�Ԫ�� | ||
| C�� | �������������ڵ��Ӳ�����Ԫ�� | D�� | �ڲ������������ԭ�� |
| A�� | ������ˮ | B�� | ���н���Ԫ�� | C�� | ˮ��Һ�ɵ��� | D�� | ����״̬�ܵ��� |
| A�� | Na��ˮ��Ӧʱ����ˮ������ | |
| B�� | Zn��25%��ϡ���ᷴӦ��ȡH2ʱ������98%��Ũ���� | |
| C�� | ��K2SO4��BaCl2����Һ��Ӧʱ������ѹǿ | |
| D�� | ��̿��ĥ��̿������ȼ�� |
| A�� | 1 mol NH5�к���5NA��N-H����NA��ʾ�����ӵ������� | |
| B�� | NH5�м��й��ۼ��������Ӽ��������ӻ����� | |
| C�� | NH5���۷е����NH3 | |
| D�� | NH5����Ͷ������ˮ�У��ɲ����������� |
| A�� | CH3-CH2-CH2-CH3 | B�� | CH3-CH�TCH-CH2-CH3 | ||
| C�� | 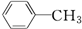 | D�� | CH3-C��C-CH3 |
| A�� | ��ԭ�ӵļ۵����Ų�ͼ��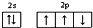 | |
| B�� | �۵����Ų�Ϊ4s24p3��Ԫ��λ�ڵ������ڵ�VA�壬��p��Ԫ�� | |
| C�� | ��ԭ����1s22s22p63s1��1s22s22p63p1ʱ��ԭ���ͷ��������ɻ�̬ת���ɼ���̬ | |
| D�� | ��һ�����ܺ͵縺�ԣ�1s22s22p4��1s22s22p3 |