��Ŀ����
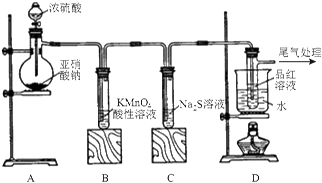
7���⻯�ƣ�CaH2�������ǵ�ɽ�˶�Ա���õ���Դ�ṩ�����⻯��Ҫ�ܷⱣ�棬һ���Ӵ���ˮ�ͷ�����Ӧ�����������ƺ��������⻯��ͨ��������������Ƽ�����ȡ��ͼ1��ģ����ȡװ�ã�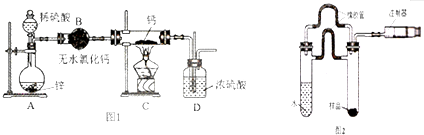
��1��װ��B�������dz�ȥ�����е�ˮ������װ��D�������Ƿ�ֹ�����е�ˮ��������Cװ�ã�
��2������ͼ1ʵ��װ�ý���ʵ�飬ʵ�鲽�����£����װ�������Ժ�װ��ҩƷ����Һ©���������ڢ٢ܢۣ��밴��ȷ��˳���������в������ţ���
�ټ��ȷ�Ӧһ��ʱ�� ���ռ����岢�����䴿��
�۹رշ�Һ©������ ��ֹͣ���ȣ������ȴ
��3��Ϊ��ȷ�Ͻ���װ��C�������Ѿ����Ӧ��B��C֮���ٽ�һװ�ã���װ���м�����Լ��ǣ���ˮ����ͭ��
��4����ͬѧ���һ��ʵ�飬�ⶨ����ʵ���еõ����⻯�ƵĴ��ȣ�����������ʵ�鲽�裮
����Ʒ�������ڼ���Na2CO3��Һ���ѧʽ�������衢���ˣ���ϴ�ӣ���������ƣ����ܺ�ɣ���������ƣ����ݳ���̼��ƣ�
��5����ͬѧ����ͼ2װ�òⶨ����ʵ���еõ����⻯�ƵĴ��ȣ�����ȡ46mg ���Ƶõ��⻯����Ʒ����¼��ʼʱע������˨ͣ����10.00mL�̶ȴ�������һ���IJ������÷�Ӧ��ȫ���к�����ȴ����˨����ͣ����57.04mL�̶ȴ�������������������ڱ�״���²ⶨ����ͨ����������Ʒ���⻯�ƵĴ��ȣ�91.3%��
���� �⻯�Ƶ��Ʊ���п��ϡ���ᷴӦ��Zn+H2SO4=ZnSO4+H2���������к���ˮ������ͨ����ˮ�Ȼ��ƽ��и����װ��C�кƽ��з�ӦCa+H2$\frac{\underline{\;\;��\;\;}}{\;}$CaH2�������⻯�ƣ����⻯����ˮ������Ӧ�����������ƺ�����������D��ֹ�����е�ˮ��������C�У�
��1���⻯��Ҫ�ܷⱣ�棬һ���Ӵ���ˮ�ͷ�����Ӧ�����������ƺ�������H2�ڷ������ȷ�Ӧ֮ǰ��Ҫ���һ������ˮ�Ȼ��ƣ���ֹ�����е�ˮ��������Cװ�ã�
��2��������μӼ��Ȼ�ȼ�յķ�Ӧ��Ҫ�����鴿��ʵ����Ϻ���Ϩ����ȴ����ֹͣ�������ɣ���ֹ����������ը��
��3�������Ƿ��������ˮ����ͭ����Ϊ��ˮ����ͭ��ˮ����ɫ��������ԣ�
��4�������ճ���̼��ƿ�֪��Ӧ����̼������Һ��ʹCaH2��Ӧ��ͬʱ�õ�̼��Ƴ�����Ȼ���ˡ�ϴ�ӡ���ɡ�������ȷ�����ȣ�
��5����ע����D��ʼʱ����ͣ����10mL�̶ȴ�����Ӧ����������ȴ����������ͣ��57.04mL�̶ȴ�����֪����������57.04mL-10mL=47.04mL����������������Ϊ��$\frac{0.04704L}{22.4L/mol}$��2g/mol=0.0042g=4.2mg�����������⻯�Ƶ�����Ϊx��������������Ϊy����Ƶ�����Ϊ46mg-x������ˮ��Ӧ������������Ϊ4.2mg-y�����ݷ���ʽCaH2+2H2O�TCa��OH��2+2H2����Ca+2H2O�TCa��OH��2+H2�����з��̼���x��y��ֵ���ٸ�����������������㣮
��� �⣺��1���⻯��Ҫ�ܷⱣ�棬һ���Ӵ���ˮ�ͷ�����Ӧ�����������ƺ�������H2�ڷ������ȷ�Ӧ֮ǰ��Ҫ���һ������ˮ�Ȼ��ƣ���װ��B�������ǣ���ȥ�����е�ˮ������װ��D�������ǣ���ֹ�����е�ˮ��������Cװ�ã�
�ʴ�Ϊ����ȥ�����е�ˮ��������ֹ�����е�ˮ��������Cװ�ã�
��2��������μӼ��Ȼ�ȼ�յķ�Ӧ��Ҫ�����鴿��ʵ����Ϻ���Ϩ����ȴ����ֹͣ�������ɣ���ֹ����������ը������ȷ�IJ���˳��Ϊ���ڢ٢ܢۣ�
�ʴ�Ϊ���ڢ٢ܢۣ�
��3�������Ƿ��������ˮ����ͭ����Ϊ��ˮ����ͭ��ˮ����ɫ��������ԣ�
�ʴ�Ϊ����ˮ����ͭ��
��4�������ճ���̼��ƿ�֪��Ӧ����̼������Һ��ʹCaH2��Ӧ��ͬʱ�õ�̼��Ƴ�����Ȼ���ˡ�ϴ�ӡ���ɡ�������ȷ�����ȣ�
�ʴ�Ϊ��Na2CO3��ϴ�ӡ���ɣ�
��5����ע����D��ʼʱ����ͣ����10mL�̶ȴ�����Ӧ����������ȴ����������ͣ��57.04mL�̶ȴ�����֪����������57.04mL-10mL=47.04mL����������������Ϊ��$\frac{0.04704L}{22.4L/mol}$��2g/mol=0.0042g=4.2mg�����������⻯�Ƶ�����Ϊx��������������Ϊy����Ƶ�����Ϊ46mg-x������ˮ��Ӧ������������Ϊ4.2mg-y����
CaH2+2H2O�TCa��OH��2+2H2��
42 4
X Y
����42��4=x��y��������y=$\frac{2x}{21}$
Ca+2H2O�TCa��OH��2+H2��
40 2
46mg-x 4.2mg-y
����40��2=��46mg-x������4.2mg-y������y=$\frac{2x}{21}$���룬���x=42mg��������Ʒ���⻯�ƵĴ���Ϊ��$\frac{42mg}{46mg}$��100%=91.30%��
�ʴ�Ϊ��91.3%��
���� �������⻯���Ʊ�Ϊ���壬����ʵ���������������������е���Ϣ�����á���ʵ��װ�õ�������������ʷ����ᴿ����ѧ���㡢ʵ�鷽����Ƶȣ��Ƕ�ѧ���ۺ������Ŀ��飬��Ŀ�Ѷ��еȣ�

 ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д� ����ͬ�����Ծ�ϵ�д�
����ͬ�����Ծ�ϵ�д�| A�� | Cu2+��NO3-��Cl-��Na+ | B�� | NH4+��Mg2+��NO3-��SO42- | ||
| C�� | K+��Ca2+��HCO3-��Cl-�� | D�� | Cl-��SO42-��K+��Na+ |
| A�� | ����ʮһ�� | B�� | ����ʮ���� | C�� | ����ʮ���� | D�� | ����ʮ���� |
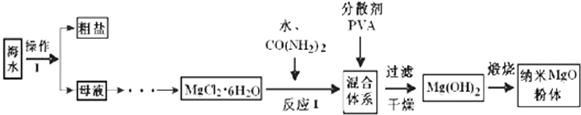
��1������I���������ᾧ�����ˣ�
��2������I��õ���ĸҺ��þ����Ũ��Ϊ1.8��10-3 mol•L-1��Ҫʹþ���Ӳ�����������Һ��pH���ӦΪ10������֪��Ksp[Mg��OH��2]=1.8��10-11��
��3����ӦI��CO��NH2��2��H20��Ӧ����C02��NH3•H20����������һ��Ҫ��ѧ��Ӧ�����ӷ���ʽΪMg2++2NH3•H20=Mg��OH��2��+2NH4+��
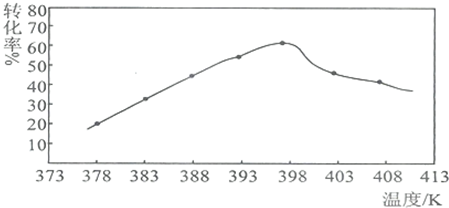
��4��ij����С���о���ӦI��378K��398Kʱ�ķ�Ӧʱ�ʡ���Ӧ������ʵ�����ȵ����ض��Ʊ���������þ���ʵ�Ӱ�죮���������ʵ����Ʊ���
| ʵ���� | T/K | ��Ӧʱ��/h | ��Ӧ������ʵ������n[CO��NH2��2]��n[MgCl2.6H2O] | ʵ��Ŀ�� |
| �� | 378 | 3 | 3��1 | ��I��ʵ��ٺ͢�̽����Ӧ������ʵ�����ȶԲ��ʵ�Ӱ�� ��II��ʵ��ں͢�̽���¶ȶԲ��ʵ�Ӱ�� ��III��ʵ��ں͢�̽����Ӧʱ��Բ��ʵ�Ӱ�� |
| �� | 378 | 4 | 4��1 | |
| �� | 378 | 3 | ||
| �� | 398 | 4 | 4��1 |
| Ԫ������ | Ԫ�ر�� | |||||||
| A | B | C | D | E | F | G | H | |
| �⻯��ķе㣨�棩 | -60.7 | -33.4 | -111.5 | 100 | -87.7 | 19.54 | -84.9 | -161.5 |
| ����ϼ� | +6 | +5 | +4 | +5 | +7 | +4 | ||
��ͻ��ϼ� | -2 | -3 | -4 | -2 | -3 | -1 | -1 | -4 |
��B��D���γɻ�����BD��BD2���������Ʊ�ǿ���ң�
��ش�
��1���������ڵ�������Ԫ�ص���ACEG���ñ���Ԫ�ر����д����д��H�����������Ľṹʽ��O=C=O��
��2���Ƚ�A��D��G���ּ������ӵİ뾶��С��rS2-��rCl-��rO2-���Ƚ�Ԫ��F��G���⻯��ķе�ߵͣ���˵������F��G�γɵ��⻯��ֱ�ΪHF��HCl�������γɵľ��嶼Ϊ���Ӿ��壬��HF����֮�����γ���������Էе�HF�ĸߣ�������ʵ�ʵ�Ԫ�ط��ű�ʾ��
��3���ɱ���DԪ�غ���Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬�������ϡ��Һ�ױ����ֽ⣬��ʹ�õĴ���Ϊ������ţ�ab��
a��MnO2 b��FeCl3 c��Na2SO3 d��KMnO4
��4���������ΪADG2��������ˮ�л�ǿ��ˮ�⣬����ʹƷ����Һ��ɫ����ɫ�����һ��ǿ�ᣮ�÷�Ӧ�Ļ�ѧ����ʽ�ǣ�SOCl2+H2O=SO2��+2HCl��
��5������˵����ȷ����bc�����ţ���
a��Ԫ��H��Ԫ��C�ĵ��ʡ����⻯����������ķе㶼��H�ĸ�
b����ҵ�ϵ���C���Ʊ�Ҫ�õ�����H��G������C������ǿ�F���⻯���ˮ��Һ��Ӧ
c����˵��Ԫ��D�ķǽ����Ա�Aǿ��ʵ�飺��D�ĵ���ͨ�뵽A���⻯���ˮ��Һ��ʵ�֣�
| A�� | ά��������ϳ������أ�ͻ�����������л���Ľ��� | |
| B�� | ����ʽΪC4H8���л�����ܴ���4��C-C���� | |
| C�� | ��CH3��3CCH2CH��C2H5��CH3 ������Ϊ2��2-����-4-�һ����飬�˴Ź���������6��� | |
| D�� | ������������Ȼ��ABS��֬�����ɸ߷��ӻ�������ɵ����� |