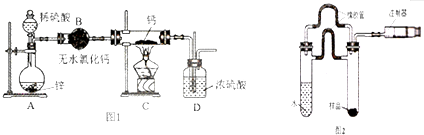
��Ŀ����
17��Ϊ�ⶨij�л�������A�Ľṹ����������ʵ�飺��һ������ʽ��ȷ��
��1�����л���A�����������г��ȼ�գ�ʵ���ã�̼������������64.86%���������������13.51%����������и�Ԫ�ص�ԭ�Ӹ�����n��C����n��H����n��O��=4��10��1
��2���������Dzⶨ���л����������ͼ��ͼ��ʾ�������ʵķ���ʽ��C4H10O
�������ṹʽ��ȷ��
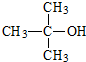
��3��A�ĺ������ͼ����C-H����O-H����C-O���������շ壮���ݼۼ����ۣ�Ԥ��A���ڴ������ʣ�����4�ֽṹ��
��4��A�ĺ˴Ź��������������壬��A�Ľṹ��ʽΪ
����������ʵ��
��5��A��ˮ������B����һ�������£�B�ɺϳ�����C����д��Bת��ΪC�Ļ�ѧ����ʽ��nCH2=C��CH3��-CH3$\stackrel{һ������}{��}$

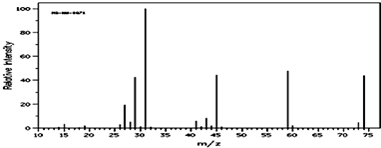
���� ��1������C��HԪ�ص����������������Ԫ�ص�����������Ȼ��������Ԫ�ص�ԭ�Ӹ���֮�ȣ�
��2����������ͼ�ж����������Ȼ����ʵ��ʽд�������ʽ��
��3��A�ĺ������ͼ����C-H����O-H����C-O���������շ壬��A�����к��й������ǻ������ڴ��࣬Ϊ������д��������ͬ���칹�壻
��4��A�ĺ˴Ź��������������壬��A�����к���2��Hԭ�ӣ��ݴ��ж���ṹ��ʽ��
��5��AΪ ���ܹ�������ȥ��Ӧ����CH2=C��CH3��-CH3��CH2=C��CH3��-CH3�����Ӿ۷�Ӧ����
���ܹ�������ȥ��Ӧ����CH2=C��CH3��-CH3��CH2=C��CH3��-CH3�����Ӿ۷�Ӧ���� ��
��
��� �⣺��1��̼������������64.86%���������������13.51%����Ԫ������������1-64.86%-13.51%=21.63%�����������̼���⡢��ԭ�Ӹ���֮��=$\frac{64.86%}{12}$��$\frac{13.51%}{1}$��$\frac{21.63%}{16}$=4��10��1��������ʵ��ʽΪ��C4H10O��
�ʴ�Ϊ��4��10��1��
��2��������ͼ��֪������Է�������Ϊ74��ʵ��ʽ��ʽ��Ϊ74���������ʽΪC4H10O��
�ʴ�Ϊ��C4H10O��
��3��A�ĺ������ͼ����C-H����O-H����C-O���������շ壬��A�����к����ǻ������ڴ������ʣ�AΪ�������������ڵ�ͬ���칹���У�CH3CH2CH2CH2OH��CH3CH2CHOHCH3����CH3��2CHOHCH3����CH3��3COH���ܹ���4�ֽṹ��
�ʴ�Ϊ������4��
��4��A�ĺ˴Ź��������������壬��A�����к���2�ֵ�ЧH����ṹ��ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��5��AΪ ���ܹ�������ȥ��Ӧ����CH2=C��CH3��-CH3��CH2=C��CH3��-CH3�����Ӿ۷�Ӧ����
���ܹ�������ȥ��Ӧ����CH2=C��CH3��-CH3��CH2=C��CH3��-CH3�����Ӿ۷�Ӧ���� ��Bת��ΪC�Ļ�ѧ����ʽΪ��nCH2=C��CH3��-CH3$\stackrel{һ������}{��}$
��Bת��ΪC�Ļ�ѧ����ʽΪ��nCH2=C��CH3��-CH3$\stackrel{һ������}{��}$ ��
��
�ʴ�Ϊ��nCH2=C��CH3��-CH3$\stackrel{һ������}{��}$ ��
��
���� ���⿼�����л������ʽ���ṹ��ʽ��ȷ������Ŀ�Ѷ��еȣ���ȷ�����л���ṹ������Ϊ���ؼ���ע����������ȷ���л������ʽ���ṹ��ʽ�ķ�����

 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�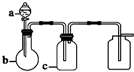 ����a��b��c��ʾ��Ӧ�����м�����Լ���������ͼ��ʾװ����ȡ���������ռ��������ǣ�������
����a��b��c��ʾ��Ӧ�����м�����Լ���������ͼ��ʾװ����ȡ���������ռ��������ǣ�������| ѡ�� | ���� | a | b | c |
| A | NH3 | Ũ��ˮ | ��ʯ�� | ��ʯ�� |
| B | CO2 | 70%ŨH2SO4 | Na2SO3���� | 95%ŨH2SO4 |
| C | NO | ϡ���� | ͭм | H2O |
| D | NO2 | Ũ���� | ͭм | NaOH��Һ |
| A�� | A�� | B�� | B�� | C�� | C�� | D�� | D�� |
| A�� | ���� | B�� | ����ȷ�� | C�� | ���� | D�� | ���� |
| A�� | baXn-�к��е�������Ϊa+b | B�� | baXn-�к��еĵ�����Ϊa-n | ||
| C�� | Xԭ�ӵ�����ԼΪ��b/NA��g | D�� | ��Ԫ�ص����ԭ������Ϊb |
| A�� | ̽���¶ȶԷ�Ӧ����Ӱ��ʱ��Ӧ�ȷֱ�ˮԡ���������������Һ��������Һ��һ���¶Ⱥ��ٻ�ϣ������Ƚ�������Һ��Ϻ�����ˮԡ���� | |
| B�� | H2O2�ڹ�������ø�Ĵ��£������¶ȵ����ߣ��ֽ����ʳ����ӿ� | |
| C�� | ʹ�ú����Ȼ��Ƶ���ѩ����ӿ��������ĸ�ʴ | |
| D�� | �ж�������Ӧ�Ƿ���ȫ����ȡ��Ӧ��Ļ��Һ������ˮ�� |
| A�� | ����ϡHNO3�м����������ۣ�Fe+4H++NO3-�TNO��+Fe3++2H2O | |
| B�� | ��ʯī���缫���MgCl2��Һ��2Cl-+2H2O$\frac{\underline{\;���\;}}{\;}$2OH-+H2��+Cl2�� | |
| C�� | NH4HCO3��Һ�м������ʯ��ˮ��HCO3-+Ca2++OH-�TCaCO3��+H2O | |
| D�� | Na2SO3��Һʹ����KMnO4��Һ��ɫ��5SO32-+6H++2MnO4-�T5SO42-+2Mn2++3H2O |
| A�� | ����о������������ϡ����������ˮ�����õ�ľ��C5H10O5�����ڶ��ǻ�ȩ��ľ����Ũ����������ÿ����ɿ�ȩ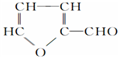 ����ľ�����ɿ�ȩ�ķ�Ӧ����ȥ��Ӧ ����ľ�����ɿ�ȩ�ķ�Ӧ����ȥ��Ӧ | |
| B�� | ȩ�ࡢ�����ǡ����ἰ�����������ܷ���������Ӧ | |
| C�� | ��˹������Ŀǰʹ����㷺����ζ������ṹ��ʽΪ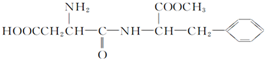 ���������ᷴӦ������Ӧ����һ�ֳ����İ����� ���������ᷴӦ������Ӧ����һ�ֳ����İ����� | |
| D�� | BAD��һ�����������ռ������Ľṹ��ʽ���£�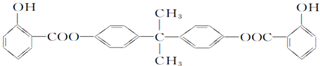 1 mol BAD����ܺͺ�6 mol NaOH����Һ���з�Ӧ 1 mol BAD����ܺͺ�6 mol NaOH����Һ���з�Ӧ |