��Ŀ����
Ϊ�����ڿ����б��ù��Ĺ������Ƶı��ʳ̶ȣ�ijѧϰС��ļס��ҡ�����λͬѧ���о����ۣ�Ϊ��Сʵ������ֹ�Э�����£��ֱ��õ����Ĺ���������Ʒ����ʵ�顣
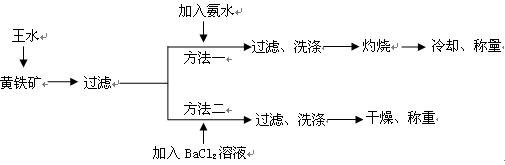
�ż�ͬѧ������ͼʵ��װ�ã�
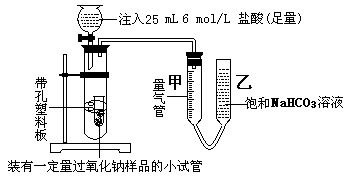
��ͬѧ��������ʱ��ʵ������У������ܼ�ǰ��ʾ����Ϊ473 mL(���۳ɱ�״���µ����)��ʵ��������ܼ����������Ҫ�ɷ��� ��
����ͬѧ������ͼʵ��װ�ã�

��ͬѧ��������ʱ��ʵ������У������ܼ�ǰ��ʾ����Ϊ249 mL(���۳ɱ�״���µ����)����ͬѧ��ʵ���У�����NaOH��Һ�������ǣ�
�� ��
�� ��
�DZ�ͬѧ��Ũ��Ϊ1.00 mol/L�ı�����ζ�����������Ʒ��Ӧѡ�õIJ��������У�
��
��ʵ��Ŀ�ķ�����ͬѧ�����õ�ָʾ��Ϊ ����ͬѧʵ����ϣ��ӵζ���ǰ���ʾ����֪���ı�����100.00 mL��
����������ʵ�����ݣ�����ù���������Ʒ���ʵİٷ���Ϊ ��ָ����ʦ�����ĸ�ʵ��С���ʵ�鱨��ʱ��ָ�����㲻�㣬��������ʵ����
�� ��
�� ��
�ż�ͬѧ������ͼʵ��װ�ã�

��ͬѧ��������ʱ��ʵ������У������ܼ�ǰ��ʾ����Ϊ473 mL(���۳ɱ�״���µ����)��ʵ��������ܼ����������Ҫ�ɷ��� ��
����ͬѧ������ͼʵ��װ�ã�

��ͬѧ��������ʱ��ʵ������У������ܼ�ǰ��ʾ����Ϊ249 mL(���۳ɱ�״���µ����)����ͬѧ��ʵ���У�����NaOH��Һ�������ǣ�
�� ��
�� ��
�DZ�ͬѧ��Ũ��Ϊ1.00 mol/L�ı�����ζ�����������Ʒ��Ӧѡ�õIJ��������У�
��
��ʵ��Ŀ�ķ�����ͬѧ�����õ�ָʾ��Ϊ ����ͬѧʵ����ϣ��ӵζ���ǰ���ʾ����֪���ı�����100.00 mL��
����������ʵ�����ݣ�����ù���������Ʒ���ʵİٷ���Ϊ ��ָ����ʦ�����ĸ�ʵ��С���ʵ�鱨��ʱ��ָ�����㲻�㣬��������ʵ����
�� ��
�� ��
��CO2��O2��N2(2�֣���һ�������1�֣������۷�))
������NaOH��Һ�е�ˮ��������Ʒ�Ӧ(1��)��ʹ��������ֻ��������������̼�������������������ɶ�����̼(1��)
���ջ�����ƿ����ʽ�ζ���(2��)������(2��)
��60%(3��)����ͬѧʵ������������Ļӷ����������ܼ����������˶�����̼�������������ƫ��(2��)����ͬѧ���к͵ζ�ʵ��ʱ������ֻ�궨һ�Σ�Ӧ�궨2~3�Σ���ƽ��ֵ����(2��)��
������NaOH��Һ�е�ˮ��������Ʒ�Ӧ(1��)��ʹ��������ֻ��������������̼�������������������ɶ�����̼(1��)
���ջ�����ƿ����ʽ�ζ���(2��)������(2��)
��60%(3��)����ͬѧʵ������������Ļӷ����������ܼ����������˶�����̼�������������ƫ��(2��)����ͬѧ���к͵ζ�ʵ��ʱ������ֻ�궨һ�Σ�Ӧ�궨2~3�Σ���ƽ��ֵ����(2��)��
��

��ϰ��ϵ�д�
�����Ŀ
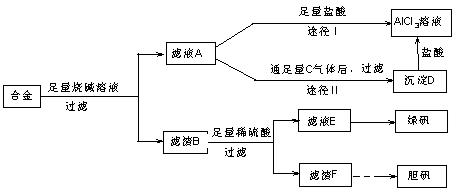
 ��
��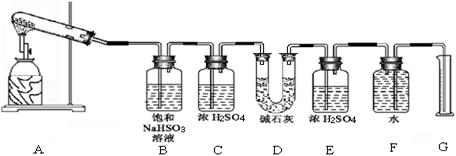
 �������ϴ����������� ��
�������ϴ����������� �� 56
56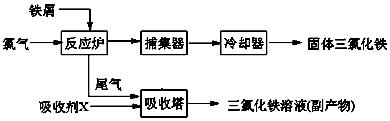
 ��(��=1.42g��cm-3)��3�����Ũ����(��=1.19g��cm-3)��϶��ɵġ�
��(��=1.42g��cm-3)��3�����Ũ����(��=1.19g��cm-3)��϶��ɵġ�
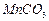 ���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϡ�ʵ������
���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϡ�ʵ������ Ϊԭ���Ʊ������ߴ�
Ϊԭ���Ʊ������ߴ� ��Һ��
��Һ��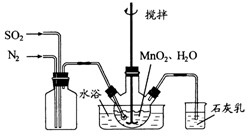
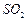 ��
��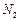 ������壬��Ӧ3h��ֹͣͨ��
������壬��Ӧ3h��ֹͣͨ�� ����
����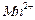 ��
��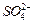 ��Ũ���淴Ӧʱ��t�仯����ͼ��������Һ��
��Ũ���淴Ӧʱ��t�仯����ͼ��������Һ��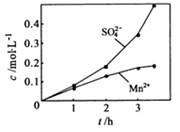
 ��ʼ����ʱ
��ʼ����ʱ ���벹���ɣ�1���Ƶõ�
���벹���ɣ�1���Ƶõ�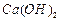 ��
��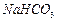 ��
��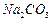 ��
��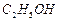 ]��
]��