��Ŀ����
��12�֣��ߴ�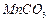 ���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϡ�ʵ������
���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϡ�ʵ������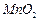 Ϊԭ���Ʊ������ߴ�
Ϊԭ���Ʊ������ߴ�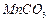 �IJ����������£�
�IJ����������£�
��1���Ʊ�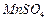 ��Һ��
��Һ��
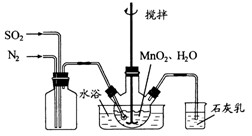
����ƿ�У�װ�ü���ͼ������һ����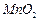 ��ˮ�����裬ͨ��
��ˮ�����裬ͨ��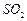 ��
��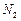 ������壬��Ӧ3h��ֹͣͨ��
������壬��Ӧ3h��ֹͣͨ��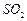 ��������ӦƬ�̣����ˣ���֪
��������ӦƬ�̣����ˣ���֪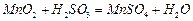 ����
����
��ʯ������뷴Ӧ�Ļ�ѧ����ʽΪ ��
�ڷ�Ӧ�����У�Ϊʹ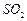 ������ת����ȫ����ͨ��
������ת����ȫ����ͨ��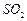 ��
��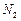 ����һ�������ı��ҺͶ�ϵ������£��ɲ�ȡ�ĺ�����ʩ�� �� ��
����һ�������ı��ҺͶ�ϵ������£��ɲ�ȡ�ĺ�����ʩ�� �� ��
����ʵ���н�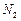 ���ɿ�������÷�ӦҺ��
���ɿ�������÷�ӦҺ��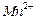 ��
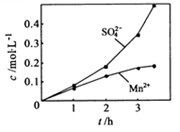
��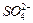 ��Ũ���淴Ӧʱ��t�仯����ͼ��������Һ��
��Ũ���淴Ӧʱ��t�仯����ͼ��������Һ��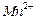 ��
��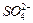 Ũ�ȱ仯�������Բ����ԭ���� ��
Ũ�ȱ仯�������Բ����ԭ���� ��
��2���Ʊ��ߴ�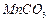 ���壺��֪
���壺��֪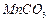 ������ˮ���Ҵ�����ʪʱ�ױ�����������100�濪ʼ�ֽ⣻
������ˮ���Ҵ�����ʪʱ�ױ�����������100�濪ʼ�ֽ⣻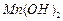 ��ʼ����ʱ
��ʼ����ʱ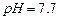 ���벹���ɣ�1���Ƶõ�
���벹���ɣ�1���Ƶõ�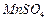 ��Һ�Ʊ��ߴ�
��Һ�Ʊ��ߴ�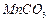 �IJ�������[ʵ���п�ѡ�õ��Լ���
�IJ�������[ʵ���п�ѡ�õ��Լ���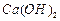 ��
��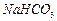 ��
��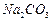 ��
��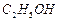 ]��
]��
�� ���� ���� ���� ���ݵ���100����
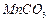 ���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϡ�ʵ������
���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϡ�ʵ������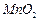 Ϊԭ���Ʊ������ߴ�
Ϊԭ���Ʊ������ߴ�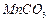 �IJ����������£�
�IJ����������£���1���Ʊ�
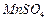 ��Һ��
��Һ��
����ƿ�У�װ�ü���ͼ������һ����
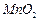 ��ˮ�����裬ͨ��
��ˮ�����裬ͨ��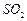 ��
��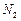 ������壬��Ӧ3h��ֹͣͨ��
������壬��Ӧ3h��ֹͣͨ��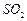 ��������ӦƬ�̣����ˣ���֪
��������ӦƬ�̣����ˣ���֪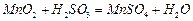 ����
������ʯ������뷴Ӧ�Ļ�ѧ����ʽΪ ��
�ڷ�Ӧ�����У�Ϊʹ
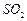 ������ת����ȫ����ͨ��
������ת����ȫ����ͨ��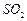 ��
��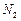 ����һ�������ı��ҺͶ�ϵ������£��ɲ�ȡ�ĺ�����ʩ�� �� ��
����һ�������ı��ҺͶ�ϵ������£��ɲ�ȡ�ĺ�����ʩ�� �� ������ʵ���н�
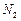 ���ɿ�������÷�ӦҺ��
���ɿ�������÷�ӦҺ��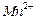 ��
��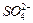 ��Ũ���淴Ӧʱ��t�仯����ͼ��������Һ��
��Ũ���淴Ӧʱ��t�仯����ͼ��������Һ��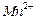 ��
��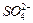 Ũ�ȱ仯�������Բ����ԭ���� ��
Ũ�ȱ仯�������Բ����ԭ���� ��
��2���Ʊ��ߴ�
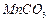 ���壺��֪
���壺��֪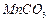 ������ˮ���Ҵ�����ʪʱ�ױ�����������100�濪ʼ�ֽ⣻
������ˮ���Ҵ�����ʪʱ�ױ�����������100�濪ʼ�ֽ⣻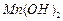 ��ʼ����ʱ
��ʼ����ʱ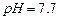 ���벹���ɣ�1���Ƶõ�
���벹���ɣ�1���Ƶõ�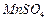 ��Һ�Ʊ��ߴ�
��Һ�Ʊ��ߴ�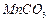 �IJ�������[ʵ���п�ѡ�õ��Լ���
�IJ�������[ʵ���п�ѡ�õ��Լ���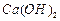 ��
��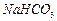 ��
��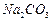 ��
��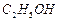 ]��
]���� ���� ���� ���� ���ݵ���100����
��

��ϰ��ϵ�д�
�����Ŀ
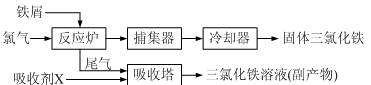
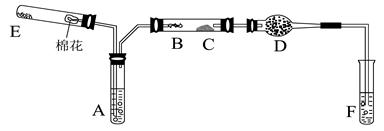
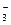 �ڲ�ͬ�����µĻ�ԭ����ϸ��ӣ���ʱ���Թ۲쵽�����������
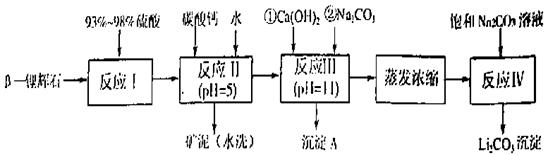
�ڲ�ͬ�����µĻ�ԭ����ϸ��ӣ���ʱ���Թ۲쵽����������� ��﮻�ʯ����Ҫ�ɷ���Li2O��Al2O3��4SiO2��������FeO��CaO��MgO�ȡ�
��﮻�ʯ����Ҫ�ɷ���Li2O��Al2O3��4SiO2��������FeO��CaO��MgO�ȡ�