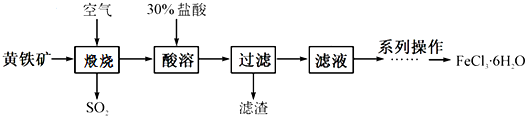
��Ŀ����
12��ij��ѧ��ȤС���ͬѧ������ͼ��ʾʵ��װ�ý���ʵ�飨ͼ��a��b��c��ʾֹˮ�У���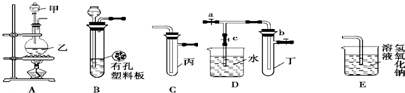
�밴Ҫ����գ�
��1������Bװ�ÿ���ȡ��������H2��CO2����2�֣���
��2��A��C��E�������װ�ÿ�������ȡCl2��������ص�����ʵ�飮���ڱ��м�������ˮ�������Ƶ���ˮ����������ˮ�ֳ����ݣ����Т�����ʵ�飬ʵ�����������������£�
| ʵ����� | ʵ����� | ���� | ���� |
| �� | ����ˮ����Ʒ����Һ | ��Һ��ɫ | ������ˮ��Ӧ�IJ�����Ư���� |
| �� | ��ˮ�м��� NaHCO3��ĩ | ����ɫ���ݲ��� | ������ˮ��Ӧ�IJ�����н�ǿ������ |
��3������������A��Cװ�����һ����ʵ�飬��֤Cl2��Br2��������ǿ�����ֱ�ָ���ס��ҡ�����ʢװ���Լ���ʵ�������ۣ��ڼס��ҡ����зֱ�װ��Ũ���ᡢMnO2��NaBr��Һ����ƿ���л���ɫ�������ɣ��Թ�����Һ����ɫ��Ϊ��ɫ��֤��Cl2��������ǿ��Br2��
��4��B��D��Eװ����������B��ʢװ����Ũ�����ͭƬ�������п����ϰ��ϣ������Ƶ�NO2�������й�ʵ�飮B�з�����Ӧ�Ļ�ѧ����ʽΪCu+4HNO3��Ũ��=Cu��NO3��2+2NO2��+2H2O������Dװ����֤NO2��ˮ�ķ�Ӧ�����������Ϊ���ȹر�ֹˮ��a��b���ٴ�ֹˮ��c��ʹ�ձ��е�ˮ�����Թܶ��IJ�����˫�ֽ��գ����ȣ��Թܶ�ʹ�Թ��������ݳ���NO2��ˮ�Ӵ��������ձ��е�ˮ�������Թܶ����Թܶ��е�NO2��һ������O2��Ϻ�ˮ�У��Թ�$\frac{9}{10}$����ˮ����ԭ�Թܶ��еĻ�������ƽ������������Ϊ42.08��44.04����������λС����
���� ��1��Bװ��Ϊ��״������ˮ�Ĺ����Һ�巴Ӧ��ȡ�����װ�ã������ü��ȣ����ݷ�Ӧ���ص������
��2��������ˮ��Ӧ���ɴ����ᣬ���������Ư���ԣ���ʹƷ����Һ��ɫ�������ӷ����Ʊ��������к���HCl���壻
��3����֤Cl-��Br-�Ļ�ԭ��ǿ���ɸ��ݷ�ӦCl2+2Br-=2Cl-+Br2���ʵ�飻
��4��Ũ�������ǿ�����ԣ���ͭ��Ӧ���ɶ����������壬NO2��ˮ��Ӧ������ɫ����NO��NO2��һ������O2��Ϻ�ˮ�У�ʣ������ΪNO���������Դ˼��㣮
��� �⣺��1��Bװ��Ϊ��״������ˮ�Ĺ����Һ�巴Ӧ��ȡ�����װ�ã������ü��ȣ���ѧ�г�������H2��CO2��NO2��NO��C2H2 H2S �ȣ�
�ʴ�Ϊ��H2��CO2��
��2��������ˮ��Ӧ���ɴ����ᣬ���������Ư���ԣ���ʹƷ����Һ��ɫ��Aװ���Ʊ��������к���HCl���壬��ʵ����û�н��г��ӣ�
HCl����ˮ������̼�����Ʒ�ĩ��Ӧ�������ݣ�����ʵ�����ۺ�����ʵ�����۲�������
�ʴ�Ϊ��ʵ�����۲���������ΪCl2Ҳ�������ԣ���ʵ����ȷ����Cl2����HClOƯ�ף�ʵ�����۲���������Ϊ��ȡ�������к���HCl���壬HCl����ˮ������NaHCO3��ĩ��Ӧ�������ݣ�
��3����֤Cl-��Br-�Ļ�ԭ��ǿ���ɸ��ݷ�ӦCl2+2Br-=2Cl-+Br2���ʵ�飬��Aװ�����Ʊ����������ɵ�����ͨ�뵽װ���廯����Һ��C�У�����ƿ���л���ɫ�������ɣ��Թ�����Һ����ɫ��Ϊ��ɫ����֤��������������ǿ���壬��Br-�Ļ�ԭ��ǿ��Cl-��
�ʴ�Ϊ���ڼס��ҡ����зֱ�װ��Ũ���ᡢMnO2��NaBr��Һ����ƿ���л���ɫ�������ɣ��Թ�����Һ����ɫ��Ϊ��ɫ��֤��Cl2��������ǿ��Br2��
��4��Ũ�������ǿ�����ԣ���ͭ��Ӧ���ɶ����������壬B�з�Ӧ�Ļ�ѧ����ʽΪCu+4HNO3��Ũ���TCu��NO3��2+2NO2��+2H2O��Dװ����֤NO2��ˮ�ķ�Ӧ��Ҫ�ȹر�a��b���ٴ�c��˫�ֽ��գ����ȣ��Թܶ�ʹ�Թ��������ݳ���NO2��ˮ�Ӵ��������ձ��е�ˮ�������Թܶ���
NO2��O2���������Թܵ�����ˮ�У�������Ӧ��4NO2+O2+2H2O=4HNO3 ���ʣ���������Ϊ������Ҳ������NO���壬��NO2��O2�����������ʵ���Ϊ10mol��
��ʣ������Ϊ��������μ�4NO2+O2+2H2O=4HNO3 ��Ӧ���ĵ�����Ϊx���������ĵ�NO2Ϊ4x������5x=9mol��x=1.8mol��������Ϊ2.8mol��V��NO2��=7.2mol��
��������������Ϊ7.2molNO2��2.8molO2������֮��Ϊ7.2mol��46g/mol+2.8mol��32g/mol=420.8g��ƽ��Ħ������$\frac{420.8g}{10mol}$=42.08g/mol��ƽ��������42.08��
��ʣ������ΪNO���壬�Թ�9/10����ˮ����ʣ��1molNO������3NO2+H2O=2HNO3+NO��˵��������NO2Ϊ3mol����Ӧ4NO2+O2+2H2O=4HNO3���ĵ����������Ϊ7mol���跴Ӧ���ĵ�����Ϊy���������ĵ�NO2Ϊ4y����5y=7mol��y=1.4mol������ʱ����Ϊ1.4mol��V��NO2��=8.6mol��
��������������Ϊ8.6molNO2��1.4molO2������֮��Ϊ8.6mol��46g/mol+1.4mol��32g/mol=440.4g��ƽ��Ħ������$\frac{440.4g}{10mol}$=44.04g/mol��ƽ��������44.04��
�ʴ�Ϊ��Cu+4HNO3��Ũ���TCu��NO3��2+2NO2��+2H2O��a��b��c��˫�ֽ��գ����ȣ��Թܶ�ʹ�Թ��������ݳ���NO2��ˮ�Ӵ��������ձ��е�ˮ�������Թܶ���
42.08��44.04��
���� ���⿼������ʵ�鷽������Ƽ�ʵ��װ�õ��ۺ�Ӧ�ã�Ϊ��Ƶ���㣬ע���Ʊ������ʵ��װ�úͷ�Ӧԭ���Լ�NO2��O2��H2O��Ӧ�ļ��㣬�������ʵ����ʼ�ʵ�鼼��Ϊ���Ĺؼ������ط�����ʵ�鼰�����������ۺϿ��飬��Ŀ�Ѷ��еȣ�

 ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д� ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д�| A�� | ��ϩˮ�����Ҵ���������ˮ�����Ҵ� | |
| B�� | �ױ�ʹ���Ը��������Һ��ɫ����Ȳ��������Ӧ��ȡ���� | |
| C�� | �ױ�������TNT����Ȳ���Ȼ��ⷴӦ������ϩ | |
| D�� | �Ҵ���ˮ����ϩ�����������廯������ϩ |
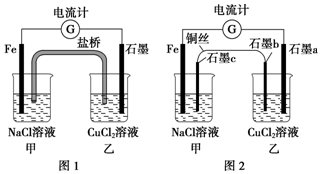 ��ͼ1��ʾװ���е����Ż���ͭ��������ʯī�����ӵõ�ͼ2��ʾװ�ã����ֵ�����ָ����Ȼ��ƫת������˵������ȷ���ǣ�������
��ͼ1��ʾװ���е����Ż���ͭ��������ʯī�����ӵõ�ͼ2��ʾװ�ã����ֵ�����ָ����Ȼ��ƫת������˵������ȷ���ǣ�������| A�� | ͼ2�е�����ָ��ƫת������ͼ1����ͬ | |
| B�� | ͼ2��ʯīa����ͭ���� | |
| C�� | ��ͼ2�м�װ��ʯīc�����μӷ�̪��Һ�����ֺ�ɫ | |
| D�� | ͼ2�е�������ΪFe�������ơ�ʯīa��ʯīb��ͭ˿��ʯīc��Fe |
����100�棬101KPa�����£�Һ̬ˮ������Ϊ40.69KJ•mol-1����H2O��g��=H2O��l�� H=+40.69KJ•mol-1
����֪25��ʱ��MgCO3��Ksp=6.82��10-6�����ڸ��¶��£����й���MgCO3����Һ�У�����c��Mg2+����c��CO32-���Ƿ���ȣ�����c��Mg2+��•c��CO32-��=6.82��10-6
����֪��
| ���ۼ� | C--C | C=C | C--H | HһH |
| ���ܣ�KJ•mol-1�� | 348 | 610 | 413 | 436 |
 ��g��+3H2��g����
��g��+3H2��g����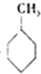 ��g����H=-384KJ•mol-1
��g����H=-384KJ•mol-1�ܳ����£���0.10mol•L-1��NH3•H2O��Һ�м�������NH4Cl���壬��NH3•H2O���뱻���ƣ���ҺpH���٣�
| A�� | �٢� | B�� | �ۢ� | C�� | �ڢ� | D�� | �٢� |
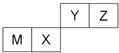
| A�� | ���Ӱ뾶Y��Z��M | |
| B�� | Y������������Ӧˮ��������Ա�X��ǿ | |
| C�� | X�������̬�⻯������ȶ��Ա�Z�Ĵ� | |
| D�� | ���ʷе㣺M��X��Y |
| A�� | 14C��������Ϊ14g•mol-1 | B�� | 14C��14N������һ����ͬ | ||
| C�� | 14C��C60��Ϊͬ�������� | D�� | 14C��12C�����ֲ�ͬ��Ԫ�� |
| A�� | Oԭ�ӷ���sp�ӻ� | B�� | Oԭ����H��Cl���γɦҼ� | ||
| C�� | �÷���Ϊֱ���ͷ��� | D�� | HClO���ӵĽṹʽ�ǣ�H-Cl-O |
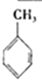
| A�� | 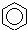 +HNO3$��_{��}^{Ũ����}$ +HNO3$��_{��}^{Ũ����}$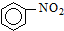 +H2O��ȡ����Ӧ +H2O��ȡ����Ӧ | |
| B�� | CH4+Cl2��CH3Cl+HCl���û���Ӧ | |
| C�� | CH2=CH2+HCl��CH3-CH2Cl���ӳɷ�Ӧ | |
| D�� | 2CH3CH2OH+O2$\stackrel{Cu}{��}$2CH3CHO+2H2O��������Ӧ |