��Ŀ����
I������ռ������Ҫ�Ļ���ԭ�ϡ�

��1��������ͼ��ʾװ�ÿɼ��֤��������̼���ռ���Һ�����˷�Ӧ����A��B���ӣ���ֹˮ�У�����ͷ�ι��е�Һ�強����ƿ����ʱ��ʵ��������___________________��
�������������䣬��A��C���ӣ��ɹ۲쵽��������__________________________��
��2����NaOH��Һ��ͨ��һ����CO2���ᾧ��õ���ɫ���壬�ð�ɫ�������ɿ����ǣ�
A��NaOH��Na2CO3��B����������������C������������������D����������������
��3�����ʵ��ȷ����2���а�ɫ�����д���A���е������ӣ�
II����ѧ��ȤС���ijƷ�������е�Ħ�����ɷּ��京����������̽����
������ϣ�������Ħ������̼��ƣ�����������ɣ������������ɷ���������ʱ�����������
������Ʒ��̼��ƵĶ����ⶨ��������ͼ��ʾװ��(ͼ�мг�������ȥ)����ʵ�飬��ַ�Ӧ
�ⶨC�����ɵ�BaCO3������������ȷ��̼��Ƶ�����������

����ʵ����̻ش��������⣺
��1��ʵ����������������ͨ������������ó��˿ɽ���B��C�еķ�Ӧ���⣬���У� ��
��2��C�з�Ӧ����BaCO3�����ӷ���ʽ�� ��
��3�����и����ʩ�У�������߲ⶨȷ�ȵ��ǣ� ��
A���ڼ�������֮ǰ��Ӧ�ž�װ���ڵ�CO2����
B���μ�����˹���
C����A��B֮������ʢ��Ũ�����ϴ��װ��
D����B��C֮������ʢ�б���̼��������Һ��ϴ��װ��
��4��ʵ����ȷ��ȡ8.00 g��Ʒ���ݣ��������βⶨ�����BaCO3ƽ������Ϊ3.94 g.����Ʒ��̼��Ƶ���������Ϊ________��
��5��������Ϊ���زⶨC�����ɵ�BaCO3������ֻҪ�ⶨװ��C������CO2ǰ��������һ������ȷ��̼��Ƶ�����������ʵ��֤�����˷����ⶨ�Ľ������ƫ�ߣ�ԭ����_________________________________��
��6��װ����U�ι�D�еļ�ʯ�ҵ�������_____________________________��

��1��������ͼ��ʾװ�ÿɼ��֤��������̼���ռ���Һ�����˷�Ӧ����A��B���ӣ���ֹˮ�У�����ͷ�ι��е�Һ�強����ƿ����ʱ��ʵ��������___________________��
�������������䣬��A��C���ӣ��ɹ۲쵽��������__________________________��
��2����NaOH��Һ��ͨ��һ����CO2���ᾧ��õ���ɫ���壬�ð�ɫ�������ɿ����ǣ�
A��NaOH��Na2CO3��B����������������C������������������D����������������
��3�����ʵ��ȷ����2���а�ɫ�����д���A���е������ӣ�
| ʵ����� | ʵ������ | ���� |
| ��ȡ������ɫ�������Թ��У�������ˮ�ܽ⣬�ټ�����BaCl2��Һ | | |
| �� | | |
II����ѧ��ȤС���ijƷ�������е�Ħ�����ɷּ��京����������̽����
������ϣ�������Ħ������̼��ƣ�����������ɣ������������ɷ���������ʱ�����������
������Ʒ��̼��ƵĶ����ⶨ��������ͼ��ʾװ��(ͼ�мг�������ȥ)����ʵ�飬��ַ�Ӧ
�ⶨC�����ɵ�BaCO3������������ȷ��̼��Ƶ�����������

����ʵ����̻ش��������⣺
��1��ʵ����������������ͨ������������ó��˿ɽ���B��C�еķ�Ӧ���⣬���У� ��
��2��C�з�Ӧ����BaCO3�����ӷ���ʽ�� ��
��3�����и����ʩ�У�������߲ⶨȷ�ȵ��ǣ� ��
A���ڼ�������֮ǰ��Ӧ�ž�װ���ڵ�CO2����
B���μ�����˹���
C����A��B֮������ʢ��Ũ�����ϴ��װ��
D����B��C֮������ʢ�б���̼��������Һ��ϴ��װ��
��4��ʵ����ȷ��ȡ8.00 g��Ʒ���ݣ��������βⶨ�����BaCO3ƽ������Ϊ3.94 g.����Ʒ��̼��Ƶ���������Ϊ________��
��5��������Ϊ���زⶨC�����ɵ�BaCO3������ֻҪ�ⶨװ��C������CO2ǰ��������һ������ȷ��̼��Ƶ�����������ʵ��֤�����˷����ⶨ�Ľ������ƫ�ߣ�ԭ����_________________________________��
��6��װ����U�ι�D�еļ�ʯ�ҵ�������_____________________________��
I����1��ˮ�ص����ɹ��ƿ������ƿ ���ƿ�еij����ܿ������ݲ���
��2��Na2CO3 Na2CO3��NaHCO3 NaHCO3
��3��
II����1�������ɵ�CO2ȫ������C�У�ʹ֮��ȫ��Ba(OH)2��Һ����
��2��Ba2++2OH-+CO2=BaCO3��+H2O ��3��C D ��4��25%
��5��B��ˮ�������Ȼ�������Ƚ���Cװ���� ��6����ֹ�����е�CO2����C
��2��Na2CO3 Na2CO3��NaHCO3 NaHCO3
��3��
| ʵ����� | ʵ������ | ���� |
| ��1�� | ������ɫ���� | ��CO32�� |
| ��2�����ˣ�ȡ������Һ���Թ��У��μӷ�̪ | ��Һ��� | ��OH�� |
II����1�������ɵ�CO2ȫ������C�У�ʹ֮��ȫ��Ba(OH)2��Һ����
��2��Ba2++2OH-+CO2=BaCO3��+H2O ��3��C D ��4��25%
��5��B��ˮ�������Ȼ�������Ƚ���Cװ���� ��6����ֹ�����е�CO2����C
���������I����1��CO2+2NaOH=Na2CO3+H2O���ڶ��߷�����Ӧ����Aװ�õ�����ѹǿ��С��Bװ���е�ˮ�ڴ���ѹ�������¾������ܽ���Aװ�á��������������䣬��A��C���ӣ��ɹ۲쵽�������ǹ��ƿ�еij����ܿ�ð���ݡ���2����NaOH��Һ��ͨ��һ����CO2�����ܷ����ķ�ӦΪ��2NaOH+CO2= Na2CO3+H2O��NaOH+CO2= NaHCO3�����Խᾧ��õ���ɫ���壬�ð�ɫ�������ɿ����ǣ�A��NaOH��Na2CO3��B��Na2CO3��C��Na2CO3��NaHCO3��D��NaHCO3����3����ȡ������ɫ�������Թ��У�������ˮ�ܽ⣬�ټ�����BaCl2��Һ��������ɫ����֤������CO32�����ӡ��ڹ��ˣ�ȡ������Һ���Թ��У��μӷ�̪����Һ���֤������OH����
II����1��ʵ����������������ͨ������������ó��˿ɽ���B��C�еķ�Ӧ���⣬���а����ɵ�CO2ȫ������C�У�ʹ֮��ȫ��Ba(OH)2��Һ���ա���2��C�з�Ӧ����BaCO3�����ӷ���ʽ��Ba2++2OH-+ CO2= BaCO3��+H2O����3��A���ڼ�������֮ǰ��Ӧ�ž�װ���ڵ�CO2������Լ��ٿ����еĶ�����̼�Բ���������Ӱ�죬��Сʵ�������ʵ���ȷ�ȡ�����B���μ�����˹�������Ƿ�Ӧ�����Ķ�����̼���屻����������Һ������գ���߷�Ӧ��ȷ�ȡ�����C����A��B֮������ʢ��Ũ�����ϴ��װ�ã�����ˮ�ֶԶ�����̼�����IJⶨ��Ȼ��Ӱ�졣��ȷ��D������B��C֮������ʢ�б���̼��������Һ��ϴ��װ�ã����ɷ�Ӧ��ӷ�������HCl���������䷴Ӧ����������̼���壬ʹ�ⶨ���ƫ����ȷ����ѡ��Ϊ��C D����4���ɷ���ʽCaCO3+2HCl=CaCl2+ CO2��+H2O; CO2+ Ba(OH)2= BaCO3��+H2O���ɵù�ϵʽCaCO3-- BaCO3����100g CaCO3��197g BaCO3.��BaCO3����Ϊ3.94 g�����Ժ��е�CaCO3������Ϊ2.0g.��Ʒ��̼��Ƶ���������Ϊ(2.0g��8.0g)��100%=25%.��5��B��ˮ�������Ȼ�������Ƚ���Cװ����.��6��װ����U�ι�D�еļ�ʯ�ҵ������Ƿ�ֹ�����е�CO2����C ,�Է�ֹӰ��ʵ��ⶨ�����ȷ�ԡ�

��ϰ��ϵ�д�
 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�
�����Ŀ


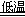 NaHCO3��+NH4Cl������ĸҺ�����ַ�����
NaHCO3��+NH4Cl������ĸҺ�����ַ�����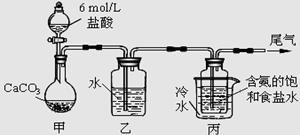
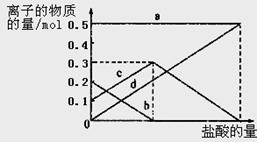
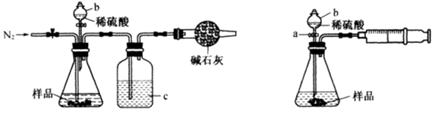
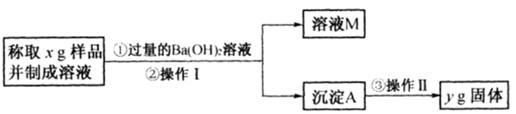
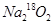 ����CO2��ȫ��Ӧ�����ù��������Ϊ( )
����CO2��ȫ��Ӧ�����ù��������Ϊ( )