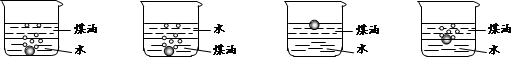��Ŀ����
���ִ���ҵ�����Ȼ���Ϊԭ���Ʊ�������ֹ����������£�

��֪NaHCO3�ڵ������ܽ�Ƚ�С��
��Ӧ��NaCl+CO2+NH3+H2O NaHCO3��+NH4Cl������ĸҺ�����ַ�����
NaHCO3��+NH4Cl������ĸҺ�����ַ�����
��1����ĸҺ�м���ʯ���飬�ɽ�����________ѭ�����á�
��2����ĸҺ��ͨ��NH3������ϸС��ʳ�ο��������£��ɵõ�NH4Cl���塣��д��ͨ��NH3���ܽ�Ƚ�С����ʽ̼����ת��Ϊ�ܽ�Ƚϴ��̼���ε����ӷ���ʽ ___________��
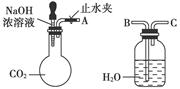
��ij��ѧС��ģ�⡰�����Ƽ������NaCl��NH3��CO2��ˮ��Ϊԭ���Լ���ͼ��ʾװ����ȡNaHCO3��Ȼ���ٽ�NaHCO3�Ƴ�Na2CO3��
��1��װ�ñ�����ˮ�������� ��
��2����װ�ñ��в�����NaHCO3��ȡNa2CO3ʱ����Ҫ���е�ʵ�������_______��ϴ�ӡ����ա�NaHCO3ת��ΪNa2CO3�Ļ�ѧ����ʽΪ ��
��3�����ڣ�2�������յ�ʱ��϶̣�NaHCO3���ֽⲻ��ȫ����С���һ�ݼ�����t1 min��NaHCO3 ��Ʒ����ɽ���������̽����
ȡ������t1 min��NaHCO3��Ʒ29��6 g��ȫ����ˮ�Ƴ���Һ��Ȼ�������Һ�л����صμ�ϡ���ᣬ�����Ͻ��衣��������ļ��룬��Һ���й����ӵ����ʵ����ı仯��ͼ��ʾ��
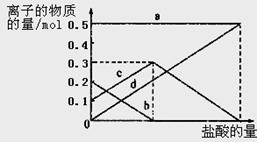
������a��Ӧ����Һ�е�������___________�������ӷ�����ͬ��������c��Ӧ����Һ�е�������___________������Ʒ��NaHCO3��Na2CO3�����ʵ���֮���� �� 21
��4����ȡ21��0 g NaHCO3���壬������t2 rnin��ʣ����������Ϊl4��8 g������Ѵ�ʣ�����ȫ�����뵽200 mL 2 mol?L��1�������У����ַ�Ӧ����Һ��H+ �����ʵ���Ũ��Ϊ____________������Һ����仯���Բ��ƣ�

��֪NaHCO3�ڵ������ܽ�Ƚ�С��
��Ӧ��NaCl+CO2+NH3+H2O
 NaHCO3��+NH4Cl������ĸҺ�����ַ�����
NaHCO3��+NH4Cl������ĸҺ�����ַ�������1����ĸҺ�м���ʯ���飬�ɽ�����________ѭ�����á�
��2����ĸҺ��ͨ��NH3������ϸС��ʳ�ο��������£��ɵõ�NH4Cl���塣��д��ͨ��NH3���ܽ�Ƚ�С����ʽ̼����ת��Ϊ�ܽ�Ƚϴ��̼���ε����ӷ���ʽ ___________��
��ij��ѧС��ģ�⡰�����Ƽ������NaCl��NH3��CO2��ˮ��Ϊԭ���Լ���ͼ��ʾװ����ȡNaHCO3��Ȼ���ٽ�NaHCO3�Ƴ�Na2CO3��

��1��װ�ñ�����ˮ�������� ��
��2����װ�ñ��в�����NaHCO3��ȡNa2CO3ʱ����Ҫ���е�ʵ�������_______��ϴ�ӡ����ա�NaHCO3ת��ΪNa2CO3�Ļ�ѧ����ʽΪ ��
��3�����ڣ�2�������յ�ʱ��϶̣�NaHCO3���ֽⲻ��ȫ����С���һ�ݼ�����t1 min��NaHCO3 ��Ʒ����ɽ���������̽����
ȡ������t1 min��NaHCO3��Ʒ29��6 g��ȫ����ˮ�Ƴ���Һ��Ȼ�������Һ�л����صμ�ϡ���ᣬ�����Ͻ��衣��������ļ��룬��Һ���й����ӵ����ʵ����ı仯��ͼ��ʾ��

������a��Ӧ����Һ�е�������___________�������ӷ�����ͬ��������c��Ӧ����Һ�е�������___________������Ʒ��NaHCO3��Na2CO3�����ʵ���֮���� �� 21
��4����ȡ21��0 g NaHCO3���壬������t2 rnin��ʣ����������Ϊl4��8 g������Ѵ�ʣ�����ȫ�����뵽200 mL 2 mol?L��1�������У����ַ�Ӧ����Һ��H+ �����ʵ���Ũ��Ϊ____________������Һ����仯���Բ��ƣ�
��1��NH3
��2��HCO3�C+NH3=NH4++CO32�C
��1����ȴ��ʹ̼�����ƾ�������
��2������ 2NaHCO3 Na2CO3+H2O+CO2��
Na2CO3+H2O+CO2��
��3��Na+ HCO3- 1:2
��4��0��75 mol/L
��2��HCO3�C+NH3=NH4++CO32�C
��1����ȴ��ʹ̼�����ƾ�������
��2������ 2NaHCO3
 Na2CO3+H2O+CO2��
Na2CO3+H2O+CO2�� ��3��Na+ HCO3- 1:2
��4��0��75 mol/L
�����������1��������NaHCO3������ĸҺNH4Cl�м������ʯ���飬�����ķ�Ӧ��Ca��OH��2+2NH4Cl=2NH3��+2H2O+CaCl2�����ղ���Ϊ�Ȼ��ơ����������а����������ã��ʴ�Ϊ��NH3��
��2��ͨ��NH3���ܽ�Ƚ�С����ʽ̼����ת��Ϊ�ܽ�Ƚϴ��̼���ε����ӷ���ʽHCO3�C+NH3=NH4++CO32�C��
��1��װ�ñ�����ˮ�������ǽ��£�ʹ̼�����ƾ���������
��2����װ�ñ��в�����NaHCO3�����ķ�ӦΪ��NH3+CO2+H2O+NaCl=NaHCO3��+NH4Cl����ȡNa2CO3ʱ��Ҫ���˵õ����壬ϴ�Ӻ�������յõ�̼���ƣ�2NaHCO3
 Na2CO3+H2O+CO2��
Na2CO3+H2O+CO2����3����Һ���й����ӵ����ʵ����ı仯Ϊ��������ʼ�ղ��䣬̼������Ӽ�С��̼���������Ũ������̼�������ȫ��ת��Ϊ̼��������ӣ��ٵ��������̼��������ӷ�Ӧ���ɶ�����̼��̼��������Ӽ�С������c���߱�ʾ����̼���������Ũ�ȱ仯��̼�������Ũ��0��2mol/L��̼���������Ũ��Ϊ0��1mol/L����Ʒ��NaHCO3��Na2CO3�����ʵ���֮����1��2�� �ʴ�Ϊ��Na+ ��HCO3-�� 1��2��
��4����ȡ21g NaHCO3�������ʵ���=21g/84g/mol=0��25mol��
������t1min��ʣ����������Ϊ14��8g�����ݻ�ѧ����ʽ���ڵ������仯���㣺
2NaHCO3=Na2CO3+CO2��+H2O ��m
2 1 62
0��2mol 0��1mol 21g-14��8g
��Ӧ��NaHCO3���ʵ���=0��25mol-0��2mol=0��05mol��NaHCO3+HCl=NaCl+H2O+CO2���������Ȼ������ʵ���0��05mol��
Na2CO3���ʵ���=0��1mol��Na2CO3+2HCl=2NaCl+H2O+CO2���������Ȼ������ʵ���0��2mol��
ʣ���Ȼ������ʵ���=0��200L��2mol/L-0��05mol-0��2mol=0��15mol��ʣ����Һ��c��H+��=
0��15mol/0��2L=0��75mol/L�ʴ�Ϊ��0��75mol/L

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ